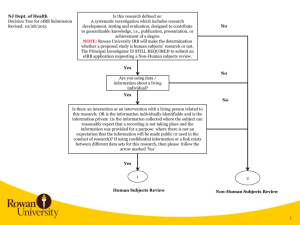
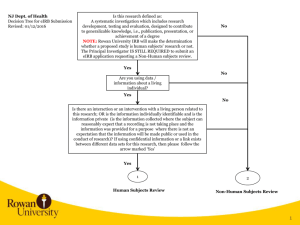
to use the eIRB Application Submission Checklist
advertisement

Institutional Review Board eIRB Submission Checklist Version date: 05/22/2015 Overview of Checklist Use the checklist when completing an eIRB application. This checklist is designed to assist an investigator in the eIRB application submission process and should not be misconstrued to represent or circumvent any Rowan University eIRB training manual or IRB guidelines, policies or procedures. Some sections below may not apply to every study. Checklist - All Rowan University personnel who will be named as the Principal Investigator, Co-Investigator(s), Study Coordinator and Study Staff Member(s) have logged into the eIRB webpage, inputted their Rowan University Network Username and Password in the login screen, and completed the eIRB registration form – eIRB Section 1.0: Study Identification - All non-Rowan University personnel who will be named as Co-Investigator(s), Study Coordinator and Study Staff Member(s) have requested an eIRB guest account via eirb@rowan.edu email and completed the guest account registration form - All personnel who will be named as the Principal Investigator, Co-Investigator(s), Study Coordinator and Study Staff Member(s) have completed Collaborative Institutional Training Initiative (CITI) training in the appropriate human subjects protections training module/learner group – eIRB Section 1.2: IRB Researcher Training Records - Completed HIPAA and Medical Research training if your research includes having direct or indirect access to patients or their health information. Note: Then all Investigators and key personnel at Rowan University who participate in the design, conduct, and/or reporting of research conducted on humans (including exempt categories of research) must complete the HIPAA and Medical Research training before IRB approval is granted. - All personnel who will be named as the Principal Investigator, Co-Investigator(s), Study Coordinator and Study Staff Member(s) are listed in the Investigator Financial and Other Personal Interest Disclosure Form, signed the form, dated the form, indicated whether or not a significant financial exists, and the appropriate Department Administration signature is obtained – eIRB Section 1.3: Conflict of Interest Note: This form is applicable to collaborators and non-Rowan University personnel identified on an eIRB application Note: If multiple Departments or Divisions are collaborating in the research, then this form needs to be signed by all Administration of the Department(s) / Home Organization(s) of the investigators. Only the investigators named on the application can be listed on the disclosure form that requires the Department signature For example, a College of Education and College of Engineering faculty collaborates on a research project. The College of Education faculty will be listed on the Investigator Financial and Other Personal Interest Form and will obtain their Department Administrator’s signature; and the College of Engineering faculty will be listed on a separate Investigator Financial and Other Personal Interest Form and will obtain their Department Administrator’s signature. Both forms will be uploaded into eIRB. - If collaborating with another Rowan University Department/College, then include that Department as an additional Department Approver – eIRB Section 1.4: Required Departmental/Division Approvers - Selected “Yes” indicating study is funded – eIRB Section 4.0: Study Funding and eIRB section 4.1: Study Funding Information Note: If the eIRB application is related to a student thesis or dissertation or if a faculty or staff study has received internal funding, then the following should be included when eIRB prompts the investigator to identify the funding: i. Institutional / Internal Funding ii. Department Funded - Identified a Rowan University study site location – eIRB Section 5.0: Study Sites Note: This should be identified for most, if not all, studies submitted to the IRB. Most studies will involve the use of Rowan University resources, which includes but is not limited to facilities to conduct meetings about the IRB project, use of computing resources and technology, and potentially, the use of facilities to conduct interventions or the implementation of the research Page 1 - Identified a non-Rowan University study site location, if applicable to the research – eIRB Section 5.01: Non-Rowan Study Sites - Uploaded the non-Rowan University study site location approval letter, signed by the non-Rowan study site authorized signer, and the letter is on the non-Rowan study site letterhead – eIRB Section 5.01: Non-Rowan Study Sites - Downloaded a Protocol Template, removed instructional and example text, provided study information in the appropriate sections in the protocol, and uploaded the completed protocol in the eIRB application – eIRB Section 7.0: Study Summary - Uploaded all research instruments; such as but not limited to screening instruments, participant assessments for capacity to consent, assessments for inclusion and exclusion criteria, surveys, interview questions, data collection sheets, and other research instruments – eIRB Section 7.0: Study Summary - Uploaded applicable recruitment material and flyers; including but not limited to verbal recruitment scripts, telephone recruitment scripts, and advertisement text and pictures for the Internet and print media – eIRB Section 11.0: Recruitment of Subjects - Uploaded the applicable consent form templates, removed instructional text, updated the appropriate sections of the template, and uploaded the applicable consent forms – eIRB Section 13.2: Consent Forms and Process of Consent - Only one (1) IRB Office contact information is listed on the consent form(s) Note: The IRB Office contact information should be the IRB of which will review the eIRB application. The IRB of record / IRB of review must correlate with the Faculty, staff or students Home Department Organization or campus/school/college of study - All documentation that is uploaded into eIRB includes a Rowan University logo or Department Letterhead/logo - All documentation uploaded into eIRB includes the Research Title - All references to the Principal Investigator in the eIRB application and all documentation uploaded that identifies the Principal Investigator includes the name of the Principal Investigator identified on the eIRB application - All documentation uploaded into eIRB, except the Investigator Financial and Other Personal Interest Disclosure Form, includes a one and half inch footer, with a version date and number on the left hand side of the footer, page number in the middle and the right hand side of the footer is left blank Note: In some instances, research materials may not be able to include a one inch footer. Please be aware that the eIRB system will stamp those documents when the review is complete. The stamp is necessary, so please make an attempt to allow any document uploaded in eIRB to include a space for the IRB approval stamp. The IRB approval stamp will be on the right-hand side of the footer section of the document(s) uploaded, regardless of whether the document does or does not include a footer. Other guidance and hints and tips for Principal Investigators submitting a Human Subjects Research Project to the IRB Office: 1) 2) 3) 4) 5) 6) 7) Students cannot be a Principal Investigator Faculty or other administrative personnel, either a full-time or ¾ time employee or faculty, needs to be assigned and identified as a Principal Investigator in the eIRB application Co-Investigators identified in the eIRB application will need to click on the ‘Accept Participation’ button located under the ‘My Activities’ section of eIRB prior to the Principal Investigator’s initial submission of the eIRB application For the initial eIRB submission, the Principal Investigator will need to click on the button titled ‘Submit Study’ located under the ‘My Activities’ section of eIRB After the initial submission, all change requests and updates to the eIRB application as a result of the IRB reviews (two reviews occur – an administrative review and an IRB member review) can be initiated by the Co-investigator using the “Submit Changes” button located under the ‘My Activities’ section of eIRB If your study already has an IRB approval from the another IRB, then you would need to submit the eIRB application as a Facilitated Review If you are submitting to the IRB as a facilitated study, for any IRB approval type of expedited or full board (convened) meeting, the Rowan University IRB will have to obtain an inter-institutional agreement with the IRB that approved the study. Please ensure that the previous study that was submitted and approved by the other IRB includes in that protocol Page 2 8) and IRB submission information and study procedures and methods associated with Rowan University. Rowan University should be identified in some form in the previous study that was approved by the other IRB. If you are unsure the proposed research is human subjects research, then please log into eIRB and submit an eIRB application to the IRB office. The application in eIRB should be marked as Non-Human Subjects Review Determination eIRB Registration guidance: 1) 2) 3) 4) Need to log into eIRB webpage and input Rowan University network username and password in login boxes on upper right of eIRB homepage Complete and input all information with a red asterick(*) next to it Faculty should identify their role as a Researcher or Investigator Students should only identify the role of Researcher or Investigator, and only input information related to their student status. Students should make the primary email account their Rowan University student email account Other webpages that are pertinent: http://www.rowan.edu/som/hsp/forms/index.html - This webpage has submission guidance, protocol template, link to consent forms, and the Investigator Disclosure Form http://www.rowan.edu/som/hsp/som-eirb/material.html - This webpage will have the eIRB Investigator and Researcher Manual and Quick Reference Guide for download and to use in the eIRB submission process https://eirb.rowan.edu/eirb - eIRB homepage to log in to view study or create eIRB profile/account http://www.rowan.edu/som/hsp/education/HIPAAandMedicalResearch.html - HIPAA and medical research training guidance Page 3