NJDOH Decision Tree for eIRB Submissions
advertisement

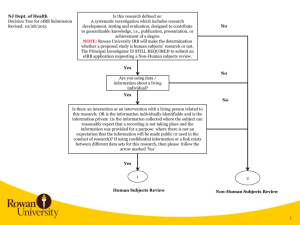
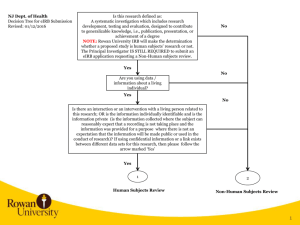
NJ Dept. of Health Decision Tree for eIRB Submission Revised: 01/25/2016 Is this research defined as: A systematic investigation which includes research development, testing and evaluation, designed to contribute to generalizable knowledge, i.e., publication, presentation, or achievement of a degree NOTE: Rowan University IRB will make the determination whether a proposed study is human subjects’ research or not. The Principal Investigator IS STILL REQUIRED to submit an eIRB application requesting a Non-Human subjects review. No Yes No Are you using data / information about a living individual? Yes No Is there an interaction or an intervention with a living person related to this research; OR is the information individually identifiable and is the information private (is the information collected where the subject can reasonably expect that a recording is not taking place and the information was provided for a purpose where there is not an expectation that the information will be made public or used in the conduct of research)? If using confidential information or a link exists between different data sets for this research, then please follow the arrow marked ‘Yes’ Yes 1 2 Human Subjects Review Non-Human Subjects Review 1 2 1 Principal Investigator requests a Guest Account in eIRB Principal Investigator, Coordinators, Co-Investigators, and Study Staff request a Guest Account in eIRB. Personnel receive notification of eIRB account in approx. 24 hours Principal Investigator and/or Co-Investigator creates eIRB application. Completes and uploads all applicable information listed in eIRB Application Requirements and completes eIRB application All Co-Investigators listed in the eIRB application need to Accept Participation. Principal Investigator receives notification of eIRB account in approx. 24 hours Principal Investigator downloads and completes Rowan University Protocol, Investigator Financial and Other Personal Interest Form and a survey or description of what will be obtained in the course of the work. For example, if a survey will be used, then that should be provided in the eIRB application. Principal Investigator logs into eIRB, creates eIRB application, uploads the applicable documentation, and completes the eIRB application. NOTE: PI needs to select the following when completing the eIRB application. On page 1.5 Review Type, the PI selects Non-Human. Principal Investigator submits study in eIRB. If errors are noted when submitting, then specific eIRB pages noted in error message need to be corrected. If no errors occur, then the study is submitted for Department Review and Approval. NJ Dept. of Health Rowan University eIRB Process eIRB Application Requirements: 1) Investigator Financial and Other Personal Interest Form 2) Protocol Template 3) Consent Forms 4) Research Materials – Data Collection Sheet, survey, interview or other instruments; screening instruments 5) Advertisements, letters 6) Detailed data that will be obtained from NJDOH via Data Use Agreement (can be listed in the protocol) 7) Investigators CV’s and resumes 8) Any other document(s)/materials pertinent to the proposed research study 9) Agreement for the Ethical Conduct of Human Subjects Research (formerly NJDOH OC-41) 3 2 3 Human Subjects Review NJ Dept. of Health (NJDOH) Rowan University eIRB Process RU IRB Administration reviews eIRB application. RU IRB determines Non-Human Subjects and issues Non-Human Subjects Determination Letter to PI RU IRB Administration reviews study and identifies any changes required. Investigator needs to upload data request in eIRB or detail the NJDOH data that will be used in the study in the protocol. Principal Investigator logs into eIRB, makes changes requested, responds to change requests, and submits changes back to RU IRB for review Non-Human Subjects Review Email the data request to NJDOH With DUA RU IRB completes review and makes a final determination. Issues Approval Letter. Without DUA RU IRB completes review and makes a final determination. Issues Approval Letter. Principal Investigator obtains fully executed NJDOH Data Use Agreement, and sends an email with the fully executed DUA attached to eIRB@rowan.edu. RU IRB uploads the fully executed DUA in the eIRB application. 3