File
advertisement

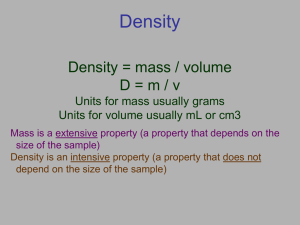
Volume is the amount of space taken up by an object Volume can also mean how much something holds Volume can be measured in many different units Volume can be calculated by using water displacement! Metric Standard Other Standards Liter (l) Milliliter (ml) Cubic Centimeter (cc or cm3) **Remember: 1 ml = 1 cc Gallon (gal.) Quart (qt.) Pint (pt.) Cup (c.) Tablespoon (tbsp.) Teaspoon (tsp.) Fluid Ounce (fl. oz.) Cubic Inches (in3 or cu in.) Cubic Feet (ft3 or cu. ft.) 1. Measure the level of the water in a container. 70 ml 60 ml 50 ml 40 ml 30 ml 20 ml 10 ml 1. Measure the level of the water in a container. 30.0 ml 70 ml 60 ml 50 ml 40 ml 30 ml 20 ml 10 ml 1. 2. Measure the level of the water in a container. 30.0 ml Insert an object in the water. We’ll use a metal ball. 70 ml 60 ml 50 ml 40 ml 30 ml 20 ml 10 ml 1. 2. 3. Measure the level of the water in a container. 30.0 ml Insert an object in the water. We’ll use a metal ball. Measure the level after the ball has displaced some of the water. 70 ml 60 ml 50 ml 40 ml 30 ml 20 ml 10 ml 1. 2. 3. Measure the level of the water in a container. 30.0 ml Insert an object in the water. We’ll use a metal ball. Measure the level after the ball has displaced some of the water. 39.0 ml 70 ml 60 ml 50 ml 40 ml 30 ml 20 ml 10 ml 1. 2. 3. 4. Measure the level of the water in a container. 30 ml Insert an object in the water. We’ll use a metal ball. Measure the level after the ball has displaced some of the water. 39 ml Finally, find the difference between the water level before displacement and after displacement. 39.0 minus 30.0 = 9.0 ml 70 ml 60 ml 50 ml 40 ml 30 ml 20 ml 10 ml 70 ml 60 ml The metal ball has a volume of 9.0 ml! 50 ml 40 ml 30 ml 20 ml 10 ml Density refers to “how crowded” the particles in an object are! Density can be measured in grams per milliliter (g/ml) M ÷ ÷ D X V 1. 2. 3. Find the volume of the object. You can use water displacement for this or traditional formulas (L x W x H). Let’s use the metal ball again. It’s volume was 9.0 ml! Now find the mass of the object. You can use a scale for this. . Mass = 54.0 g Divide the mass by the volume! 54.0 g ÷ 9.0 ml = 6.0 g/ml Pure water has a density of 1.00 g/ml If any material is denser than the fluid that surrounds it, it will ___________ If any material is less dense than the fluid that surrounds it, It will __________! SINK or FLOAT In Water (D = 1.0 g/mL) Float Float Float Sink Sink Sink (alcohol) Float (fuel) Float Check out this picture Which layer has the highest density? Which layer has the lowest density? Imagine that the liquids have the following densities: 10g/cm3. 6g/cm3. Which 3g/cm3. 5g/cm3. number would go with which layer? Imagine that the liquids on the right have the following densities: 15g/cm3 3g/cm3 7g/cm3 10g/cm3 9g/cm3 12g/cm3 the colors to the correct densities. 3g/cm3 7g/cm3 9g/cm3 10g/cm3 Match 12g/cm3 15g/cm3