Photosynthesis SL
advertisement

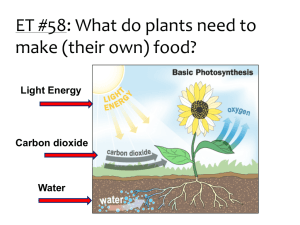
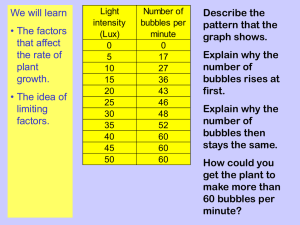
Photosynthesis Topic 3.8 Key points….. Photosynthesis involves conversion of LIGHT energy (usually from the sun) into CHEMICAL energy (in food). (Microsoft Clip art) The overall equation: Carbon Dioxide + Water + Light → Glucose + Oxygen 6CO2 + 6H2O + Energy → C6H12O6 + 6O2 Sunlight consists of WHITE light but is actually made up of a range of wavelengths (colours) including red, blue and green. http://mirror-in-bom1.gallery.hd.org/_exhibits/natural-science/prism-and-refraction-of-light-into-rainbow-AJHD.jpg http://quarknet.fnal.gov/quarknet-summer-research/QNET2010/Astronomy/em_spectrum.jpg More key points…. Pigments in the leaves can absorb light. The main photosynthetic pigment is CHLOROPHYLL. (There are a few others too, such as the carotenoids.) Chlorophyll absorbs red and blue light well, but reflects green light. This is why leaves appear green. Some of the energy absorbed by chlorophyll is used to produce ATP. Absorption Spectra show how much light is taken in by pigments. In chlorophyll this light energy is used to drive photosynthesis. This is shown in the Action Spectra which shows how much Photosynthesis reactions take place http://www.life.illinois.edu/govindjee/paper/fig5.gif Some of the energy absorbed by chlorophyll is used to split water molecules into oxygen and hydrogen. This is called PHOTOLYSIS. H+ H H O H+ Light Energy O2- The hydrogen is used later on in the process of photosynthesis. The oxygen is released as a waste product. Lets look at the equation for Photosynthesis again……. 6CO2 + 6H2O + Energy → C6H12O6 + 6O2 Where do these “C’s” come from? Where do these “O’s” come from? What about these “O’s”? Where do this “H’s” come from? The carbon is used to make glucose initially, and a range of organic substances. Conversion of carbon in a gas (CO2) to carbon in a solid (glucose) is called CARBON FIXATION. Carbon fixation involves the use of hydrogen from photolysis and energy from ATP. The leaf is the organ responsible for photosynthesis. So how do all the necessary ingredients for Photosynthesis meet up in the leaf? http://s2.hubimg.com/u/746729_f520.jpg Carbon dioxide is absorbed into the leaf via the stoma / stomata. Oxygen leaves by the stoma. http://www.emc.maricopa.edu/faculty/farabee/biobk/leafstru.gif Water comes up from the roots via tubes called Xylem Glucose that is produced is taken to the rest of the plant in the phloem tubes. Look at the picture of the leaf structure on the previous slide – then fill in the labels on this one A- Air space http://www.bioclix.org/images/leaf_2.jpg B- Spongy mesophyll C- Vascular bundle D- Palisade layer E- Stomata Your task: In the next 3 slides think about what you would have to do to measure the Rate (speed) of Photosynthesis. Draw how you would set up the apparatus (equipment) for the 3 different ways of measuring the rate mentioned. Measuring rate of Photosynthesis Production of oxygen Aquatic plants (eg. Elodea) give off bubbles of oxygen during photosynthesis. These bubbles can either be counted or collected and the volume measured to give an indication of rate. Production of Oxygen Experiment Measuring rate of photosynthesis 2 Uptake of carbon dioxide Leaves absorb carbon dioxide from the air or water, but this is difficult to measure directly. CO2 is an acidic gas, so when it is removed from water by photosynthesis, the pH rises. This can be measured with indicators and pH meters. Uptake of Carbon Dioxide Experiment Measuring rate of photosynthesis 3 Increase in biomass Biomass is the biological mass of organisms. If batches of plants are harvested at different times, and the biomass is determined, the rate of increase in biomass gives an indirect measure of rate of photosynthesis. Increase in Biomass Experiment What affects how fast Photosynthesis occurs? Why and how does it affect it? Amount of CO2 and H2O. If you don’t have raw materials……….. Light Intensity You need energy to make the chemical reactions happen……. Temperature Photosynthesis needs enzymes…….. Draw and annotate graphs to explain each. Production of oxygen set up http://moodle.schoolnet.lk/file.php/21/assets/images/ks4/elodea.gif You could either count the number of bubbles in a set amount of time or measure the volume of gas produced over a longer period of time. Sodium Hydrogen Carbonate provides CO2 to the plant. Back Uptake of Carbon Dioxide set up http://c.photoshelter.com/img-get/I0000xQykazfHOUU/s Put an aquatic plant in the water with a known amount of CO2 dissolved in it. Monitor the pH levels (using a pH probe) over time to measure CO2 uptake. pH should increase with time. Rate of change of pH is a measure of Rate of P/S. Back Increase in Biomass set up Weigh the dry aquatic plant, carry out the experiment and weigh the dry plant again. % change in biomass over time can be a measure of Rate of P/S. (Microsoft Clip art) Back