3.3 Understanding gas pressure and atmospheric pressure
advertisement

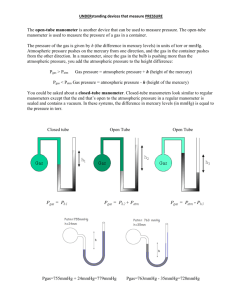
Existence of Gas pressure based on the kinetic theory gas molecules move freely and randomly. The gas molecules collide with one another and also collide with the walls of their container. The collision of gas molecules with one another is an elastic collision The collision of gas molecules with the wall of the container produces change of momentum or impulsive force So the gas molecules exert a pressure on the inside of the container because pressure is force per unit area (P=F) A ……………………………………………………………………………………….. The conclusion about gas pressure Gas pressure is the force per unit area exerted by the gas molecules as they collide with the walls of their container. What happen if the volume inside a container decreases? The rate of collisions between the gas molecules with the walls of the container increase Therefore the pressure of the gas increases ……..Volume decreases, pressure increases…….. ( what law ??????) What happen if the container is heated? The velocity of the gas molecules increase The kinetic energy of the gas molecules increase The collision rate between the gas molecules with the walls of container increase So the force of collisions will increase Therefore the pressure of the gas increases …..Temperature increases, pressure increases… ( what law???????) Measuring Gas Pressure 1.Manometer manometer P gas = P atmosfera + h Pgas = Patmosfera - h P gas = P atmosfera Example 1 Calculate the pressure of the gas supply in the units (i) cm Hg (ii) Pa [ Density of mercury = 1.36 x 104 kg m-3 and Atmospheric pressure = 76 cm Hg ] 2. Bourdon Gauge Bourdon gauge The another use of U-Tube The U-tube can also be used to determine the density of a liquid. Pressure P1 = Pressure P2 h11g = h22g h11 = h22 Example 2 The figure shows a U-tube use to determine the density of a liquid K. When liquid K is poured into one arm, the water level in the other arm rises. If the density of water is 1 000 kg m-3, determine the density of liquid K . Existance of Atmosheric The gas molecules in the air possess mass and experience the pull of gravity. The result is that gases have weight. The weight of the gas molecules will produce force and as a result will exert pressure on you because pressure is force per area ( P = F ) A The pressure is called the pressure. atmospheric Characteristics of Atmospheric Pressure acts equally in all directions pressure on any object is not dependent on the surface area of the object. influenced by the altitude. (Hence as the altitude increases , the atmospheric pressure decreases because the higher it is from the surface of the Earth , the lower is the density of air.) -The value of atmospheric pressure at sea level is approximately 1 atm = 1x 105 Pa = 76 cm Hg = 10 m of water. Example 3 The atmospheric pressure is 76 cm Hg. Calculate the atmospheric pressure in the units Pa. [Density of mercury = 1.36 x104 kg m-3 ]. Measuring atmospheric pressure The Simple Fortin barometer The mercury column rises or falls according to the pressure of air on the mercury in the dish. The space above the mercury column is a vacuum so it exerts no pressure on the top of the mercury column. If the vertical height of the mercury is h cm , therefore the atmospheric pressure reading is “ h cm mercury ”. BAROMETER THINK !!!!! What happen to a column of mercury if a barometer is taken to the top of a mountain and why? What happen to a column Of mercury if a barometer is taken to the bottom of the sea and why? Example 4 The figure shows a mercury barometer is placed in a school laboratory where the atmospheric pressure is 75 cm Hg. If the density of mercury is 1.36 x 104 kgm-3 and the density of water is 1 x 103 kgm-3, determine (i) the atmospheric pressure in the units Pa (ii) the value of h if the mercury is replaced by water. (iii) the value of h if the barometer is submerged in water at depth of 40.8 cm. Aneroid Barometer When the atmospheric pressure decreases , the container will expand. When the atmospheric pressure increases, the container will constrict. The slight movement of the box is magnified by a lever system which is connected to a pointer. The Aneroid barometer can be used as an altimeter by mountaineers or in an aeroplane to determine its altitude. BAROMETER ANEROID BAROMETER ANEROID Applications of Atmospheric Pressure (i) Drinking straw (ii) Rubber sucker (iii) Syringe THE END