File - Respiratory Therapy Files
advertisement

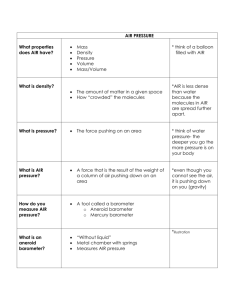
Pressure Week 3 Definition of Pressure Defined as force (F) acting perpendicularly to a surface area (A) P = F/A In the atmosphere the gas we breathe (Nitrogen, Oxygen, Co2 and trace gases) are in constant motion, creating kinetic energy and FORCE applied against the surface of the earth. This is the atmospheric pressure Atmospheric pressure Atmospheric pressure is usually measured by the height of a liquid in a closed, evacuated tube as shown on pages 178-179 in the text book. You have already learned the normal values at sea level for one atmosphere in several different pressure scales. At altitudes above sea level the atmospheric pressure is lower because there is less air pushing down on the surface. At sea level, 1 ATM = 760 torr, In Denver, 1 mile above sea level, 1 ATM = 680 tor. Atmospheric pressure units At sea level: ◦ ◦ ◦ ◦ ◦ ◦ ◦ 760 mmHg 760 torr 1034 cmH2O 33 ft H2O 14.7 PSI 29.9 inHg 101.33 kPa ◦ *in respiratory we will use mmhg/torr Positive pressure Pressure applied that is above the current atmospheric pressure In respiratory we do this all the time with devices such as mechanical ventilators We do this to overcomes a resistance or disruption in normal pressure gradients between the atmosphere and the patients lung Negative pressure "Negative" pressure is a gauge pressure that is below atmospheric pressure. Since atmospheric pressure measured in gauge pressure is 0, any gauge pressure that is below atmospheric pressure will be negative. It is very important to remember that if you are measuring pressure on an absolute scale, you can not have a pressure that is less than 0 or a negative number. Negative is also called vacuum pressure We use Negative pressure when suctioning patients. For example: a suction pressure of 60 mmHg is a vacuum, or 60 less that the current atmospheric pressure The air is made up of molecules. Gravity pulls the air molecules toward the earth, giving them weight. The weight of the air molecules all around us is called the air pressure. Your weight is the result of gravity pulling your mass down on the bathroom scales. Note that weight has units of a force, such as pounds. High altitudes = lower pressure Low altitudes = higher pressure Atmospheric pressure Air pressure can be thought of as the column of air rising above us. As we go up in altitude, we get closer to the top of the column. Thus there are fewer molecules of air above us to be pulled down by gravity, so the air “weighs” less. Therefore, pressure always decreases as one goes up. Atmospheric Pressure Gas pressure depends on both density and temperature. Adding air molecules increases the pressure in a balloon. Heating the air also increases the pressure. Air pressure is equal in all directions. Because air is a fluid, force applied in one direction is distributed equally in all directions. Thus the downward pull of gravity on air molecules produces air pressure in all directions. Pressure = force per unit area As elevation goes up Barometric pressure goes down. This is an inverse relationship. A Barometer is used to measure air pressure. Torricelli’s barometer used a glass column suspended in a bowl of mercury. The pressure of the air molecules pushed the mercury up into the glass tube. The weight of the mercury in the tube was equal to the weight of the air pressing down on the mercury in the dish. As atmospheric pressure increases… The mercury in the tube rises. The Mercury Barometer Good: Bad: •Simple to construct •Glass tube is fragile •Highly accurate •Mercury is very toxic! Although mercury has been used for hundreds of years, its toxic effects have only been fully realized in the last few decades. Students should NEVER handle mercury or broken mercury thermometers or barometers. Mercury should also never be thrown in the trash or washed down the drain, since it moves easily up the food chain from fish to humans. The Aneroid Barometer •No fragile tubes! •No toxic chemicals! •No batteries! •Never needs winding! MILLIBARS An aneroid barometer uses a cell which has had most of the air removed. As the air pressure around the cell increases, it presses on the cell, which causes the needle to move. Television weather forecasters usually give barometric pressure in inches of mercury. However, meteorologists measure atmospheric pressure in millibars. Most aneroid barometers have a needle which can be set to remember the previous reading. Atmospheric Pressure Although pressure varies with altitude, the concentration of gases do not change. Meaning there is 21% O2 and 78% Nitrogen everywhere in the atmosphere. The difference is the pressure. Less pressure= less pressure change from in the lung to the outside. High altitudes = increased work of breathing Changing Pressure A rising barometer = increasing air pressure. This usually means: Rising barometer readings indicate that a high pressure system is approaching. Higher atmospheric pressure is usually associated with fair weather and clearing skies. Changing Pressure A falling barometer = decreasing air pressure. This usually means: Falling barometer readings usually indicate the approach of an area of low pressure. Low pressure readings are usually associated with storm systems. Tornadoes and hurricanes can produce very low barometric readings. Air Movement and Flow 6-1 flow from areas of high Fluids (air and water) pressure to areas of low pressure. Change in pressure across a horizontal distance is a pressure gradient. Greater the difference in pressure and the shorter the distance between them, the steeper the pressure gradient and the high the velocity of flow In the lung, flow travels to the alveoli based on the diameter of the airway and also the pressure gradient. We will learn about this in another lecture Pressure gradient in lung speed, velocity, and flow. Speed is a measurement of an object’s movement in units of distance or length /time, (ie cm/sec, miles per hour). Velocity is the speed of an object with its direction at a given instant, (ie 50 miles/hour South). Fluid flow is a special kind of velocity expressed in units of volume/time, ie ml/sec, l/min. Remember that a fluid is a substance capable of flowing ---a gas or liquid. Kinetic Theory of Matter The Kinetic Theory of Matter is the theory that all molecules are in constant motion resulting in kinetic energy. This theory applies to all three states of matter -- solids, liquids and gases. States of Matter Solids – have a high degree of internal order; their atoms have a strong mutual attractive force Liquids – atoms exhibit less degree of mutual attraction compared with solids, they take the shape of their container, are difficult to compress, exhibit the phenomenon of flow Gases – weak molecular attractive forces; gas molecules exhibit rapid, random motion with frequent collisions, gases are easily compressible, expand to fill their container, exhibit the phenomenon of flow States of Matter All matter possesses energy. There are 2 types of internal energy: The energy of position, and the energy of motion. Internal energy of matter • Potential energy (Position) The strong attractive forces between molecules that cause rigidity in solids • Kinetic energy (Motion) Gases have weak attractive forces that allow the molecules to move about more freely, interacting with other objects that they come in contact with Internal energy and temperature • The two are closely related: internal energy can be increased by heating or by performing work on it. •Absolute zero = no kinetic energy Physical properties of a solid: Possess the least amount of KE Mostly Potential Energy in intermolecular forces holding particles together Can maintain their volume & shape Physical properties of a liquid: Intermolecular, cohesive forces are not as strong They exhibit fluidity (particles sliding They exhibit a buoyant force Essentially incompressible Assume the shape of their container Physical properties of a gas: Extremely weak – if any – cohesive forces Possess the greatest amount of KE & the least amount of Potential Energy Motion of atoms & molecules is random Do not maintain their shapes & volumes but expand to fill the available space Exhibit the phenomenon of flow Exhibits the least thermal conductivity Uses: Gas therapy (Oxygen, Heliox, Nitrous oxide…HHN/SVN…) Change of State Liquid-solid phase changes (melting and freezing) Melting = changeover from the solid to the liquid state Melting point = the temperature at which melting occur. Freezing = the opposite of melting Freezing point = the temperature at which the substance freezes; same as its melting point 38 Change of State (cont.) Properties of liquids ◦ Pressure – depends on the height and weight density. ◦ Buoyancy – occurs because the pressure below a submerged object always exceeds the pressure above it ◦ Viscosity – the force opposing a fluid’s flow. The greater the viscosity of a fluid, the greater the resistance to flow. Blood has a viscosity five times greater than that of water 39 Change of State (cont.) Heat transfer ◦ Conduction – transfers heat in solids ◦ Convection – transfers heat in liquids and gases (Example: heating homes or infant incubators) ◦ Radiation – occurs without direct contact between two substances - example: microwave oven ◦ Evaporation/Condensation: requires heat energy to occur ◦ Sublimation - change from a solid to a gas without an intermediate change to a liquid - example dry ice turning into CO2 http://www.youtube.com/watch?v=lxPDAh4je54 40 Heat Transfer Conduction: http://www.youtube.com/watch?v=9UxU0ELgYO A Convection http://www.youtube.com/watch?v=oFRMvVNia00 &feature=relmfu Radiation http://www.youtube.com/watch?v=RmFJOVOia_4 &feature=relmfu Condensation/evaporation http://www.youtube.com/watch?v=QjSfIDARTik&f eature=related Change of State (cont.) Pascal’s Principle. Liquid pressure depends only on the height and weight density of the liquid and not the shape of the vessel or total volume of a liquid. 42 Pascal's law Pascal's law states that pressure exerted anywhere in a confined incompressible fluid is transmitted equally in all directions throughout the fluid such that the pressure ratio (initial difference) remains the same. Pascal’s Law states that when you apply pressure to confined fluids (contained in a flexible yet leak-proof enclosure so that it can’t flow out), the fluids will then transmit that same pressure in all directions within the container, at the same rate. The simplest instance of this is stepping on a balloon; the balloon bulges out on all sides under the foot and not just on one side. This is precisely what Pascal’s Law is all about – the air which is the fluid in this case, was confined by the balloon, and you applied pressure with your foot causing it to get displaced uniformly. Change of State (cont.) Cohesion and adhesion ◦ The attractive force between like molecules is cohesion. ◦ The attractive force between unlike molecules is adhesion. The shape of the meniscus depends on the relative strengths of adhesion and cohesion. H20: Adhesion > Cohesion Mercury: Cohesion > Adhesion H20 Mercury 44 Cohesion and Adhesion Cohesion: Water is attracted to water Adhesion: Water is attracted to other substances Adhesion and cohesion are water properties that affect every water molecule on earth and also the interaction of water molecules with molecules of other substances. Essentially, cohesion and adhesion are the "stickiness" that water molecules have for each other and for other substances. The water drop is composed of water molecules that like to stick together, an example of the property of cohesion. The water drop is stuck to the end of the pine needles, which is an example of the property of adhesion. Notice I also threw in the all-important property of gravity, which is causing the water drops to roll along the pine needle, attempting to fall downwards. It is lucky for the drops that adhesion is holding them, at least for now, to the pine needle. http://www.youtube.com/watch?v=VHnFMPxteGo Change of State (cont.) Liquid to vapor phase changes ◦ Boiling – heating a liquid to a temperature at which its vapor pressure equals atmospheric pressure. ◦ Saturation – equilibrium condition in which a gas holds all the water vapor molecules that it can. ◦ Dew point – temperature at which the water vapor in a gas begins to condense back into a liquid. ◦ Evaporation – when water enters its gaseous state at a temperature below its boiling point. 46 The Gas Law Ideal Gas follows kinetic molecular theory, made up of large number of molecules that are in rapid random motion following perfect elastic collitions losing no momentum How the Kinetic Molecular Theory Explains the Gas Laws The pressure of a gas results from collisions between the gas particles and the walls of the container. Each time a gas particle hits the wall, it exerts a force on the wall. An increase in the number of gas particles in the container increases the frequency of collisions with the walls and therefore the pressure of the gas. Avogadro's Hypothesis As the number of gas particles increases, the frequency of collisions with the walls of the container must increase. This, in turn, leads to an increase in the pressure of the gas. Flexible containers, such as a balloon, will expand until the pressure of the gas inside the balloon once again balances the pressure of the gas outside. Thus, the volume of the gas is proportional to the number of gas particles. The Gas Laws Charles Law ◦ The volume of a gas increased with the temperature ◦ The volume of a given amount of dry ideal gas is directly proportional to the Kelvin Temperature provided the amount of gas and the pressure remain fixed. ◦ When we plot the Volume of a gas against the Kelvin temperature it forms a straight line. ◦ V1 / T1 = V2 / T2 Boyle’s Law ◦ the product of the pressure and volume are observed to be nearly constant. ◦ The product of pressure and volume is exactly a constant for an ideal gas. ◦ p * V = constant WATER VAPOR (9.8oC)