Air Around You Power Point
advertisement

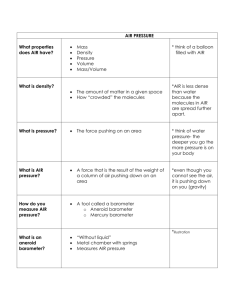
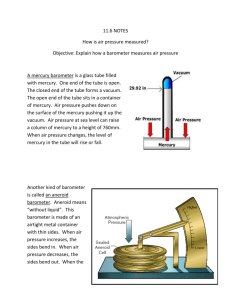
The Air Around You Earth’s Atmosphere • Earth’s atmosphere is the envelope of gasses that surround the planet. • Nitrogen makes up 78% of our air • Oxygen makes up 21% of our air • The remaining 1 % are called trace gasses. • How many more times Nitrogen than Oxygen in our air? • Read about nitrogen and oxygen on page 7-8 in your book and answer questions 4-8 in your notes. Water Vapor • Water in the form of a gas is called water vapor • Water vapor is not the same as steam because steam is warm air with tiny droplets of water in it. • What role does water vapor play in Earth’s weather? How does Earth’s atmosphere make conditions on Earth suitable for living things? • Oxygen and other gases needed for life • Constantly moving in and out of living things • Warmth • Liquid water • Protects from radiation and meteoroid Do the next page in your notes (pg. 50) on your own as review. What you don’t get done is homework. When you are finished, begin to read section two (Air Pressure). Properties of Air • Weight of the atmosphere is constantly pushing on your body • Air has mass because it is composed of atoms and molecules. • Because air has mass, it has density and pressure • The more molecules in a given volume of air, the greater its density. Cause and Effect • If mass increases and volume stays the same then density increases • If mass decreases and volume stays the same then density decreases • If mass stays the same and volume decreases then density increases • If mass stays the same and volume increases then density decreases Measuring Air Pressure • An instrument used to measure air pressure is a barometer. • Mercury barometers have liquid mercury forced up a column when air pressure increases • Aneroid barometer has no liquid and has thin walls and air tight metal chamber that bulges when air pressure increases. Mercury Barometer Aneroid Barometer 1644- Evangelista Torricelli 1843- Lucien Vidie Read pp. 12-13 Aneroid = “without fluid” Units of Air Pressure • Most weather reports for the general public use inches of mercury. For example, if the column of mercury in a mercury barometer is 30 inches high, the air pressure is 30 in. • National Weather Service maps indicate air pressure in millibars. One inch of mercury is approximately 33.87 millibars. Isobars are lines on maps that join places that have the same air pressure. Altitude • Another word for elevation, or distance above sea level • At the top of a mountain the air pressure is less than the air pressure at sea level. • Air pressure decreases as altitude increases. • As air pressure decreases, so does density. • Read “Altitude Affects Air Pressure” on pg. 13 to answer why air pressure is greater at sea level than at the top of a mountain. Altitude also affects density • As you go up through the atmosphere the density of air decreases. • The gas molecules that make up the atmosphere are farther apart at higher altitudes. • If you were near the top of a tall mountain and began to run, you would run out of breath more quickly than at sea level. Why? – The air contains 21% Oxygen at any level, but since the air is less dense at higher altitudes there are fewer oxygen molecules to breath in each cubic meter if air than at sea level. • Explain why mountain climbers sometimes bring tanks of oxygen along with them on their climbs. Do page 57 and 58 for homework **Do not give up on page 58! Be sure to read the directions first. They will help you find the right answers!**