Osmosis and Diffusion

O
SMOSIS AND
D
IFFUSION
By: Raelee Robinson and Chantal Jabbour
I
NTRODUCTION TO OSMOSIS AND
DIFFUSION
Diffusion
Osmosis
All living things have certain requirements they must satisfy in order to stay alive
A cell membrane allows some substances to leave or enter the cell.
Diffusion plays a part in moving substances into and out of a cell
Concentration determines the direction that a substance takes through the cell membrane
Particles move from areas of higher concentration to areas of lower concentration until there is a balance – “equilibrium”
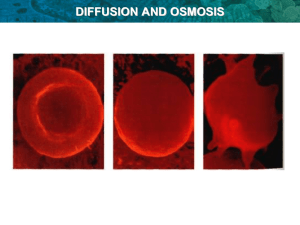
The diffusion of water through a selectively permeable membrane is called Osmosis
L
ESSON
S
EQUENCE
Diffusion
The movement of a substance from an area of high concentration to an area of low concentration
Diffusion is a passive process where substances move across a concentration gradient from high to low
The process of diffusion creates equilibrium
Molecules are in constant motion
Lesson Sequence
Osmosis
A special type of diffusion
Occurs when water molecules move across a partially permeable membrane (like a cell membrane) from a lower concentrated solution of a higher concentrated solution
Solute particles are too large to pass through the membrane, so the water moves
Lesson Sequence
Diffusion and Osmosis in plant and animal cells
Cell membranes are selectively permeable
Solutions can be classified as hypotonic, hypertonic, and isotonic relative to their solute concentrations
Water potential describes the tendency of water to leave one area in favour of another, it is affected by 2 factors: pressure and amount of solute (use examples of this in red blood cells, as well as potato cells)
CURRICULUM EXPECTATIONS
Grade 8: Understanding Life Systems: Cells
Overall Expectations:
2. investigate functions and processes of plant and animal cells;
3. demonstrate an understanding of the basic structure and function of plant and animal cells and cell processes.
Specific Expectations:
3.3 compare the structure and function of plant and animal cells
3.4 explain the processes of diffusion and osmosis and their roles within a cell
T
EACHING
A
PPROACH
Video demonstration on Diffusion and Osmosis
T
EACHING
A
PPROACH
Gizmo activity on diffusion . Students explore the motion of particles as they bounce across the screen and through an adjustable gap. Temperature, mass and initial number of particles can be altered.
Lab Activity
Investigation of Diffusion and Osmosis Using Eggs
Prior to the lab, the teacher will dissolve the shells of 4 eggs using vinegar so that only the membrane remains on the egg to create a simulation of a cell.
Materials:
Eggs (see above)
Water
Water coloured with blue food colouring
Molasses
Corn syrup
4 beakers
Toothpicks
Procedure:
Students will determine the mass of the eggs and record it in a table
They should pour 150 ml of each substance into it’s own beaker, and then add an egg to each beaker and store in the fridge for 24 hours
After 24 students remove the eggs from their beakers and record their
observations. Students should record the volume of liquid in the beaker as well as the mass of the egg
After recording their observations, students should use a toothpick to pop the egg membrane and record their observations
Safety
Students should wear safety goggles while popping the egg membrane with a toothpick
The lab floor area should be cleared of all backpacks, stools etc
The teacher should ask students if there are any allergies to the materials being used in the lab
See teacher notes below, taken from sciencespot.net
P
OTENTIAL
S
TUDENT
D
IFFICULTIES
Difficulty understanding the abstract concept of molecules and how they permeate a cell membrane
Visuals including the Gizmo activity, web video animations, and lab activity may help the students to conceptualize the membrane’s qualities
Difficulty with the concept of solutions’ “desire” to reach equilibrium
The teacher can demonstrate this by using simple familiar demonstrations such as food colouring in water
Difficulty applying and using vocabulary such as hypotonic vs. hypertonic, solute, solvent, concentration gradient etc correctly
Students will have plenty of exposure to the correct use of vocabulary through the Gizmo, and multimedia introduced.
They will practice their vocabulary and knowledge during the lab activity to identify the hypertonic and hypotonic solutions
A Word Wall with the words and their definitions can be used for easy student reference
R
EAL
W
ORLD
A
PPLICATIONS
Diffusion
How odours travel through air
Pollution and air quality
Mixing of substances
Osmosis
Water passing through plants from their roots
Cooking with water
Osmosis in medicine (red blood cell, kidneys)
Preservation of fruits and meets
Water Filters
D
IFFERENTIATED
A
SSESSMENT
Students will work in partners and choose one of the following activities for their final assessment
Interpersonal:
Find someone who uses diffusion or osmosis in their career and conduct an interview asking them questions related to osmosis or diffusion and their job
Linguistic:
Write a diary or computer in some way to journal entry of a create a demonstration of water molecule going diffusion. The entry should describe the chart must be included process, and the highlighting the similarities differences between
Linguistic:
Create a poem or mind map describing osmosis and diffusion, be sure to use as much unit vocabulary as possible.