Play Presentation
advertisement
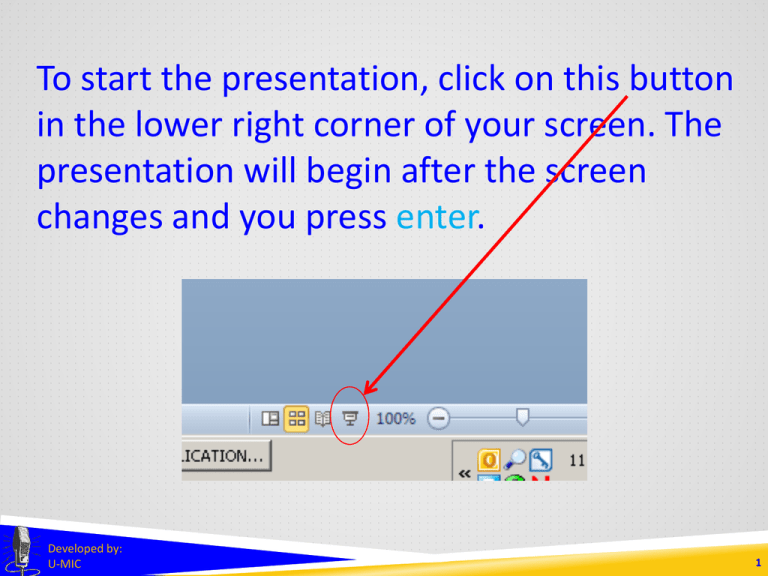
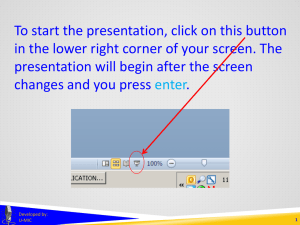
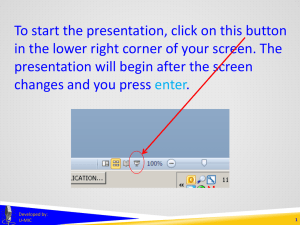
To start the presentation, click on this button in the lower right corner of your screen. The presentation will begin after the screen changes and you press enter. Developed by: U-MIC 1 EXEMPTIONS 1, 2, AND 2a in human subjects research Developed by: U-MIC University of Michigan IRB Collaborative Exemptions 1, 2, and 2a Certain sub-categories of research • exempt from federal regulations governing IRB operations • exempt under UM’s flexibility initiative • • no greater than minimal risk not federally funded not FDA-regulated Exempt research does not require • • ongoing IRB oversight through continuing review specific approval of processes or consent materials Developed by: U-MIC 3 Exemptions 1, 2, and 2a UM policy requires IRB staff or the Office of Research to confirm exemption eligibility via a streamlined eResearch application. Exempt determinations do not require review by • • convened IRB expedited review Developed by: U-MIC 4 Exemptions 1, 2, and 2a Research • • on educational methods based on survey and interview methodologies may be eligible for • federal exemption category 1 • federal exemption category 2 • UM flexibility initiative exemption category 2a Developed by: U-MIC 5 Exemptions 1, 2, and 2a Federal exemption category 1 Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (i) research on regular and special education instructional strategies, or (ii) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods. Education settings • most often schools • any place where instruction takes place Normal educational practices • activities that typically occur in a classroom or other educational setting • • • • test development experimentation with instructional methods evaluation of classroom or school activities assessment of attitudes about learning or curriculum Developed by: U-MIC UMHS Clinical Simulation Center mannequin 6 Exemptions 1, 2, and 2a Federal exemption category 2 Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: (i) information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects; and (ii) any disclosure of the human subjects’ responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation. If particularly sensitive, data must be recorded anonymously. Data collection methods are limited to • educational tests • surveys, interviews, focus groups • observation of public behavior Examples: • web-based or in-person surveys of adults • study drawing on Twitter posts Developed by: U-MIC 7 Exemptions 1, 2, and 2a Research not eligible for exemption 2 • survey/interview studies involving minors • studies involving physical or behavioral interventions Developed by: U-MIC 8 Exemptions 1, 2, and 2a University of Michigan flexibility initiative exemption category 2a • • • minimal risk adults non-invasive intervention • • • • • • • video story economic game computer program experimental tool exposure to stimuli data collection via • • • • • survey interview (including focus groups) test observation physiological measurements Developed by: U-MIC 9 Exemptions 1, 2, and 2a Research not eligible for exemption 2a • more than minimal risk • federal funding • other regulatory or contractual restrictions • • FDA-regulated components NIH Certificate of Confidentiality Developed by: U-MIC 10 Exemptions 1, 2, and 2a Exemption categories 1, 2, and 2a References http://www.irb.umich.edu/application/exempt.html http://medicine.umich.edu/medschool/research/office-research/institutional-reviewboards/guidance/exempt-research http://www.hhs.gov/ohrp/policy/faq/exempt-research-determination/index.html http://www.irb.umich.edu/education/downloads/IRB-HSBS_Newsletter_Spring_2013.pdf Developed by: U-MIC 11 THANK YOU. Lark Speyer IRBMED Cindy Shindledecker IRB-HSBS Developed by: U-MIC 12