Play Presentation - HRPP
advertisement

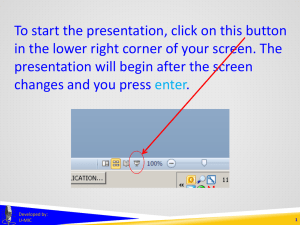
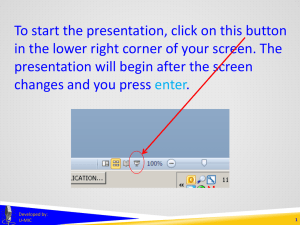
To start the presentation, click on this button in the lower right corner of your screen. The presentation will begin after the screen changes and you press enter. Developed by: U-MIC 1 DOCUMENT REVIEW AND FINALIZATION at scheduled continuing review Developed by: U-MIC University of Michigan IRB Collaborative document review and finalization review of supporting documents • initial review • scheduled continuing review (while documents are in use) recruitment • after enrollment ends, IRB no longer reviews recruitment materials at SCR Developed by: U-MIC informed consent • after subject interaction and intervention are concluded, IRB no longer reviews consent documents at SCR 3 document review and finalization recruitment materials • IRB reviews recruitment materials at SCR, if: • enrollment ongoing • eResearch renewal application section 1.1 • eResearch renewal application section 1.1.1 • IRB updates documents’ approval and expiration dates • If enrollment has permanently ended • X-DO NOT USE_ • IRB does not review recruitment materials at SCR Developed by: U-MIC 4 document review and finalization informed consent documents • IRB reviews consent documents at SCR, if: • subject interaction/intervention ongoing or planned • eResearch renewal application section 1.1 • eResearch renewal application section 1.1.1 • IRB updates documents’ approval and expiration dates • If further study activities are limited to follow-up • X-DO NOT USE_ • IRB does not review recruitment materials at SCR Developed by: U-MIC 5 document review and finalization multisite trials coordinated by another site UM IRB does not review documents once they are no longer in use at UM coordinated by the University of Michigan UM IRB may continue to review documents even after they are no longer in use at UM • depends on UM’s role as coordinating site Developed by: U-MIC 6 document review and finalization IRB review of supporting documents at scheduled continuing review Developed by: U-MIC 7 THANK YOU. Brian Seabolt IRBMED Developed by: U-MIC 8