Play Presentation - HRPP
advertisement

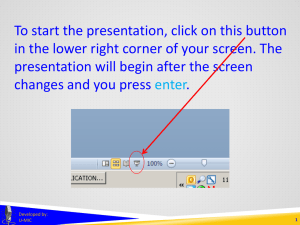
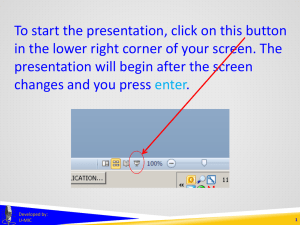
To start the presentation, click on this button in the lower right corner of your screen. The presentation will begin after the screen changes and you press enter. Developed by: U-MIC 1 UMBRELLA PROJECTS Developed by: U-MIC University of Michigan IRB Collaborative Umbrella projects OHRP IRB review not required for projects lacking immediate plans for involvement of human subjects Developed by: U-MIC 3 Umbrella projects Certain types of applications and proposals lack definite plans for the involvement of human subjects either because the specific human subject activities have not yet been fully developed or because human subject research was not anticipated at the time of the application. In such cases, the application or proposal need not be reviewed by the IRB prior to an award (see 45 CFR 46.118 and 46.119). OHRP memo, 2000 “IRB Review of Applications for HHS Support” Developed by: U-MIC 4 Umbrella projects Certain types of applications and proposals lack definite plans for the involvement of human subjects either because the specific human subject activities have not yet been fully developed or because human subject research was not anticipated at the time of the application. In such cases, the application or proposal need not be reviewed by the IRB prior to an award (see 45 CFR 46.118 and 46.119). OHRP memo, 2000 “IRB Review of Applications for HHS Support” Developed by: U-MIC 5 Umbrella projects Certain types of applications and proposals lack definite plans for the involvement of human subjects either because the specific human subject activities have not yet been fully developed or because human subject research was not anticipated at the time of the application. In such cases, the application or proposal need not be reviewed by the IRB prior to an award (see 45 CFR 46.118 and 46.119). OHRP memo, 2000 “IRB Review of Applications for HHS Support” Developed by: U-MIC 6 Umbrella projects Certain types of applications and proposals lack definite plans for the involvement of human subjects either because the specific human subject activities have not yet been fully developed or because human subject research was not anticipated at the time of the application. In such cases, the application or proposal need not be reviewed by the IRB prior to an award (see 45 CFR 46.118 and 46.119). OHRP memo, 2000 “IRB Review of Applications for HHS Support” Developed by: U-MIC 7 Umbrella projects Certain types of applications and proposals lack definite plans for the involvement of human subjects either because the specific human subject activities have not yet been fully developed or because human subject research was not anticipated at the time of the application. In such cases, the application or proposal need not be reviewed by the IRB prior to an award (see 45 CFR 46.118 and 46.119). OHRP memo, 2000 “IRB Review of Applications for HHS Support” Developed by: U-MIC 8 Umbrella projects granting agencies • • request documentation of IRB approval before releasing funds may request approval documentation even for research that will not directly or immediately involve human subjects training grant program project center grant project that will eventually involve human subjects http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html#46.118 Developed by: U-MIC 9 Umbrella projects umbrella projects or dry applications Developed by: U-MIC 10 Umbrella projects Developed by: U-MIC 11 Umbrella projects expedited review Developed by: U-MIC 12 Umbrella projects umbrella applications that do anticipate a human subjects research component that do not anticipate a human subjects research component must receive IRB approval before starting research may be able to terminate after grant is finalized • • amend umbrella application to become a standard application or create a separate standard application Developed by: U-MIC 13 Umbrella projects Contact the IRB for more information about umbrella projects. Developed by: U-MIC 14 THANK YOU. Lark Speyer IRBMED Developed by: U-MIC 15