SSS 2 PHYSICS 2ND POWER POINT PRESENTATION
advertisement
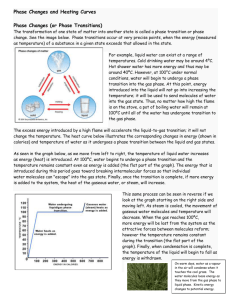
SSS TWO PHYSICS FIRST TERM DEFINITION OF TERMS Boiling point of a substance is defined as the temperature at which its saturated vapour pressure becomes equal to the external atmospheric pressure Relative humidity is the ratio of the mass of water vapour in a given volume of air to the mass of water vapour required to saturate the same volume of air at the same temperature. It is expressed in percent. It is measured with a hygrometer. It is used by weathermen for making weather forecast. Evaporation is the escape of water molecules into the atmosphere from a water contained body. It causes cooling. Factors affecting evaporation Temperature Surface Wind Humidity/dryness of air Pressure Nature of liquid/viscosity/density Factors affecting boiling point of a liquid Nature of the liquid External atmospheric pressure Presence of impurities Degree of its molecular cohesive force N/B The extra heat giving to boiling water is not to increase the temperature but to break the intermolecular forces in the molecules of water. Differences between boiling and evaporation Evaporation This is the change from liquid to vapour at temperature below normal boiling point. Takes place slowly at the liquid surface. Takes place at all temperatures. Boiling This is the change from liquid to vapour at the boiling point. Occurs throughout the entire volume of the liquid. Takes place at a particular temperature. Differences between boiling and evaporation Evaporation Boiling • Temperature needs • Temperature not be steady remains steady during evaporation. during boiling. Wind assists Wind has no effect evaporation. on boiling. The similarity between evaporation and boiling is that both involve liquid phenomenon. Assignments The mass of water vapour in a given volume of air at 20 degree Celsius is 0.05g. The air requires 0.15g of water vapour to saturate it at the same temperature. Calculate the relative humidity of the air. Describe an experiment to show that evaporation causes cooling. Define heat capacity and state its unit. A quantity of pepper soup of mass 800g poured into a plastic container with a tight fitting lid has a temperature of 30 degree Celsius. The container is then placed in a microwave oven, rated 1200W and operated for 3minutes. Calculate the final temperature attained by the soup. (Assuming no heat losses). Explain why containers with tight-fitting lids are not suitable for use in microwave cooking. When the soup is brought out and allowed to cool, a dent is observed on the container. Explain. ( Take specific heat capacity of the soup = 4000J/kgK)