Three States of Matter
advertisement

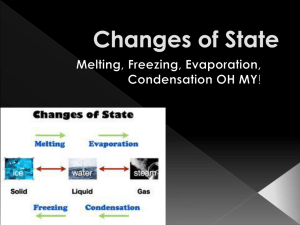
Three States of Matter Beta Science Overview In this powerpoint you will be introduced to three states of matter and you will explore the similarities and differences between these states. Vanishing Act Pour rubbing alcohol into a small plastic cup until the alcohol just covers the bottom of the cup Moisten the tip of a cotton swab by dipping it into the alcohol in the cup. Rub the cotton swab on the palm of your hand. Make sure there are no cuts or abrasions on your hands. Record your observations. “Vanishing Act” Analysis 1.) Explain what happened to the alcohol after you rubbed the swab on your hand. 2.) Did you feel a sensation of hot or cold? If so, how do you explain what you observed? States of Matter States of Matter: the physical forms in which a substance can exist. Ex. Water commonly exists in three states of matter: solid (ice), liquid (water), and gas (steam) Particles of Matter Matter is made up of tiny particles called “atoms” and “molecules.” They are too small to see without a microscope They are always in motion and bumping in to one another. Because they bump into each other, they are always interacting. The way they interact with one another determines the state of matter they become. Particles of a SOLID Particles of a solid do not move fast enough to overcome the strong attraction between them. So, they are close together and vibrate in place. Particles of a LIQUID Particles of a liquid move fast enough to overcome some of the attraction between them. The particles are close together but can slide past one another. Particles of a GAS Particles of a gas move fast enough to overcome almost all of the attraction between them. The particles are far apart and move independently of one another. http://www.youtube.com/watch?v=s-KvoVzukHo Solids Solid: the state of matter that has a definite shape and volume. Particles stay close together The attraction between them is stronger than the attraction between the particles of the same substance in the liquid or gaseous state The particles move but not fast enough to overcome their attraction with each other. Each particle vibrates in place so they actually become locked in place by the particles around themselves Two Kinds of Solids Crystalline solids: have a very orderly, three dimensional arrangement of particles. Particles are in a repeating pattern of rows. Ex. Iron, diamond, ice Amorphous solids: made of particles that do not have a special arrangement. Each particle is in one place but not in any particular pattern. Ex. Glass, rubber, wax Liquids Liquid: the state of matter that has a definite volume but takes the shape of its container. Particles in liquids move fast enough to overcome some of the attractions between them. Particles slide past each other until they take the shape of their container. Although liquids change shape, they do not easily change volume. Ex. 50ml of water will take up the same space in a graduated cylinder as it would in a beaker. Liquid Characteristics Surface tension: a force that acts on the particles at the surface of a liquid. Causes some liquids to bead up Different liquids have different surface tension Viscosity: a liquid’s resistance to flow. The stronger the attraction of particles, the more viscous the liquid is. Gases Gas: is the state of matter that has no definite shape or volume. Particles move quickly so they can break away from one another. There is less attraction between particles of a gas than between particles of the same substance in the solid or liquid state. The amount of empty space between gas particles can change. Ex. Helium in a tank compared with helium in a balloon.