4.0 The particle theory
advertisement

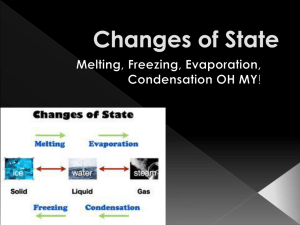
THE PARTICLE THEORY ACTIVITY What are the states of matter? Make Oobleck- how does this fits into the states of matter? STATES OF MATTER There are 4 main states of matter 1. 2. 3. 4. Solid Liquid Gas Plasma THE PARTICLE THEORY There are four main points: 1. 2. All matter is made up of tiny particles that are too small to see These particles are always moving and vibrating. Solid particles move the least, and gas particles move the most 3. The particles of matter are all attracted or bonded to each other 4. The particles have spaces between them. Solid matter have small spaces than liquids and gases ACTIVITY Complete what’s the total volume and mix it up again and compare the results. We will discuss your results after the activity HOW THE PARTICLE THEORY EXPLAINS MIXING SUBSTANCES Particles in matter are attracted to each other, which is why solids are so rigid However particles can also be attracted to other substances more than to themselves This explains why substances can dissolve When salt is added to water, the salt particles are more attracted to the water and the salt particles break apart and spread out We will be learning more about dissolving DISCUSSION 1. If you added the marbles to a beaker of sand would you get the same results? Why or why not? DISSOLVING Many solutions are made by dissolving solids into a liquid Ex. Sugar dissolves in water and it appears to have ‘disappeared’. This means that it is water soluble In solutions the solute is more attracted to the water than it is to itself resulting in the solid separating Do all solutes act this way? Think back to adding flour or pepper to water, what happened? If the particles are more attracted to itself than it will remain a solid and remain a mixture DISSOLVING Can you speed up dissolving? If I were to give you a sugar cube, brainstorm some methods that would speed up how fast that sugar cube dissolved. RATE OF DISSOLVING The particle theory helps to explain the rate of dissolving 1. Stirring- stirring moves particles around, this causes the solvent particles to bump into the solute particles breaking the bond between solute particles 2. Small solutes dissolve quicker than large Demo- sugar cube vs sugar crystals 3. Increasing the temperature- particles move faster when they heat up and the solute and solvent will bump into each other more often CHECK YOUR PROGRESS Answer the questions and hand them in