Experiment: Cold Packs & Enthalpy of Solution
advertisement

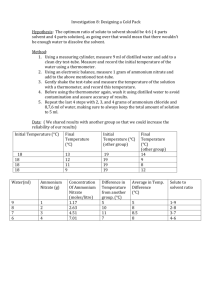
Studying calorimetry using cold packs What if the dissolution is endothermic? The dissolution of ammonium nitrate absorbs heat. Write the thermochemical equation for the dissolution of ammonium nitrate. HEAT Water & Calorimeter CH 113 (M.Hall) Ch6 1 Experiment: Cold Packs & Enthalpy of Solution Objective The objective of the experiment is to determine the enthalpy of solution for the compound found in cold packs, ammonium nitrate. Apparatus Coffee cup calorimeter with cover (CCal = 25 J/°C) Top loading balance Weigh boats (small) LabPro with temperature probe Magnetic stirrer and stir bar NH4NO3(s) Chemicals Deionized water Ammonium nitrate CH 113 (M.Hall) Ch6 2 Data & Observations 1. Determine the mass of a small weigh boat. Add to it approximately 8 g of ammonium nitrate and record the mass again. 1. Record the mass of the clean, dry coffee cup calorimeter. Bring a beaker containing ~100 mL of deionized water to the balance with you. 3. Fill the calorimeter about 2/3 full with deionized water. Record the mass of the calorimeter and water. 4. Place the calorimeter with its lid and a stir bar into a 250 mL beaker. Set this up with the magnetic stirrer and the LabPro + temperature probe. Start stirring the water such that the stir bar does not hit the temperature probe. 5. Set the LabPro to read temperature. 6. Record the initial temperature of the water (Ti). 7. Quickly add the ammonium nitrate to the water in the calorimeter, taking care not to get any solid on the sides of the cup. Replace the lid. 8. As the mixture stirs, record temperatures every 10-15 seconds (exact timing does not matter). You are looking for a temperature extreme. After the temperature passes an extreme, record the temperature every minute or so for about 10 minutes. 9. In the meantime, mass the weigh boat to determine exactly how much lithium chloride actually ended up in the coffee cup calorimeter. CH 113 (M.Hall) Ch13 3 Experiment: Cold Packs & Enthalpy of Solution Name____________________________ Partner___________________________ Section _____ Calculations 1. Is the dissolution process for ammonium nitrate “the source” or “the sink?” 2. What was the source of the heat? 3. Calculate the heat for the dissolution of ammonium nitrate, qdiss. 1. For the dissolution of ammonium nitrate in this experiment, DHdiss ≠ qdiss. Why not? 1. Calculate DHdiss. 2. Calculate the percent error in the experimental value for DHdiss. CH 113 (M.Hall) Ch13 4 Error and its Source The calorimeter is not a perfect insulator. How would this limitation affect the final temperature of the solution? How would this source of error affect the DHdiss.? How could you adjust the experiment to minimize this source of error? We used an average value for the heat capacity of the calorimeter. Are all calorimeters identical? How would this source of error affect the DHdiss.? How could you adjust the experiment to minimize this source of error? Was your experimentally determined DHdiss value too high or too low? Account for this error using the sources above and/or other sources. 5