Ammonium nitrate is a chemical that is widely used
advertisement

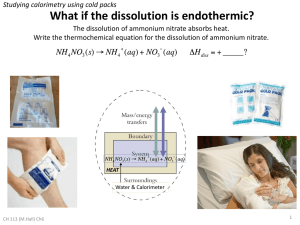
Experimental Background Ammonium nitrate is a chemical that is widely used by gardeners as a fertilizer. It is a white powdery substance that can be very dangerous if not handled correctly. It is not usually found in a crystallized state unless it is being used for an experiment. Because it is used in creating explosive agents, it is classified as an oxidizing agent (Workplace Health and Safety Queensland.) An oxidizing agent is an agent that gives away its oxygen element(s) to another element creating a chemically bonded compound. This chemical is used in explosives because of its chemical properties. It has a boiling point of 210 degree Celsius and a melting point of 170 degrees Celsius. The boiling point is when the chemical becomes dangerous in its use. As it heats up, it starts to expand rapidly and soon explodes. It has a pH of 4.0 making it acidic and flammable (Ammonium.) One of the biggest issues with Ammonium nitrate is its use in Afghanistan as an explosive. In the form of fertilizer, the chemical is used to create bombs that have led to the death hundreds of US troops in Afghanistan. Due to this, troops have started trading farmers for their Ammonium nitrate fertilizer in return for money (Pike.) Terrorists in other areas of the world have also made explosives with ammonium nitrate. It reacts with fuels in a fast manner causing the gas to heat up and explode. Many bombs have been made with this knowledge (Nanjappa.) It is also not a banned substance due to its wide variety of use, even though the government has tried to make it illegal using the Explosives Substance Act (Economic Times.) In past experiments, ammonium nitrate has been used to create small reactions that cause instant flames or freezing. In one experiment, a certain amount of water is added to a beaker. Then the temperature of the water is recorded and ammonium nitrate is added. The purpose of the experiment is to see the affect of the ammonium nitrate on the temperature of the water. This experiment is an example of an endothermic reaction because the temperature of the water decreases significantly (United Nuclear.) Because ammonium nitrate is highly reactive, it can be used with ammonium chloride and Zinc dust to create a flame. This mixture can be extremely flammable if it is in any contact with water (United Nuclear.) The purpose of this experiment is to observe the affect of the change in temperature of a water and ammonium nitrate mixture when the amount of ammonium nitrate is dissolved. To carry out this experiment, 30g of ammonium nitrate, 1g distilled water, 10 beakers of 100ml, and a thermometer will be used. In 10 beakers, there will be 75ml of distilled water. The temperature of the water in each beaker will be taken and recorded. After this, a fixed amount of ammonium nitrate will be dissolved into each beaker of water. After the ammonium nitrate is completely dissolved, the temperature of the water will be taken and recorded again for each mixture. This process will be repeated with different amounts of ammonium nitrate being dissolved into the water. The results of the temperature changes will be observed, recorded, and then analyzed. Experimental Results After three trials were done for each amount of Ammonium nitrate mixed with water, the results of the temperatures were averaged into bar graphs. The graph below shows the first few trials of three grams averaged into final results. Affect of Ammonium Nitrate on Temperature of Water Temperature in Degrees Celsius 25 20 15 Series1 Series2 10 5 0 1 2 3 Grams of Ammonium Nitrate (3) Figure 1 In the first part of the experiment, the 175ml of water was an average of 23.3 degrees Celsius before the Ammonium nitrate was added. After the compound was added, the temperature dropped an average of 1.3 degrees Celsius as seen in Figure 1. After the changes of the three grams of Ammonium nitrate were tested, four grams of Ammonium nitrate were prepared to be mixed into the water. The results of these trials are shown in Figure 2. In these trials, the average temperature of water was 21.5 degrees Celsius. The graph shows the drop in temperature after the compound was added. The average drop in temperature was 1.5 degrees Celsius. The next amount of Ammonium nitrate added was five grams. Once all of the Ammonium nitrate was measured out and everything was set up, the temperature of the water was recorded. The results are shown in Figure 3. The average temperature of the water in these trials was 21.6 degrees Celsius. The average drop in temperature after the Ammonium nitrate was dissolved into the water was 2.2 degrees Celsius. Discussion Once the graphs were created, the results showed that the Ammonium nitrate did drop the temperature of the water as predicted. It was a slight change that occurred in every trial. As the amount of the compound increased, the drop in temperature increased as well. Some days, the starting temperature of the water was lower than the starting temperature of other days. This factor was ruled out in the averaging of the change in temperature from before and after. This showed that the more grams of the compound are added, the greater affect it will have on the final temperature of the water.