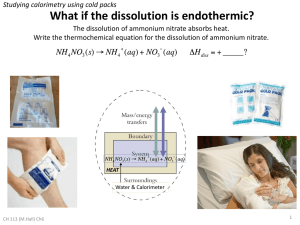
Postlab 8 1. Use data from the 4 calibration trials to calculate the average heat capacity, Ccal, of your calorimeter. Use a Grubbs test to identify outliers. Calculate the standard deviation and 95% confidence interval using the remaining data points. - Ccal (kJ/Celsius) Trial 1- 372.18 Trial 2- 305.78 Trial 3- 328.76 Trial 4- 314.98 Sample Calculation- 2. Use data from the ammonium nitrate dissolution experiment to calculate the heat of solution in kJ/mol. Use a Grubbs test to identify outliers. Calculate the standard deviation and 95% confidence interval using the remaining data points. -Ccal (kJ/Celsius) Trial 1- 0.31 Trial 2- 0.67 Trial 3- 0.073 Trial 4- 0.32 3. Look up the literature value for the heat of solution of ammonium nitrate and compare this with your measured value. What was your % error? What are likely causes for this deviation in your result? The literature value of ammonium nitrate is 25.7 kJ/mol was much higher than the calculated value. The % error is above 100 which shows that there is a huge error in the data set. It could have been better to record the minimum temperature right away rather than waiting for a long period of time. 4. Calculate the average efficiency of your calorimeter (% of heat absorbed by the water in the calorimeter relative to the amount of heat produced by dissolving the ammonium nitrate) including 95% confidence limits. Use the literature value for the heat of solution. For the purpose of this calculation, neglect the heat absorbed by the Thermos®. Efficacy= 1.45% Mean= 1.267 Standard deviation = 0.127 95% CI = 1.267±0.0876 (±7.12%) 5. Write an abstract for this experiment. An abstract is a brief (one paragraph or less) description of the goal and results of the experiment. Using a calorimeter and certain known values, such as the specific heat of water, the experimenters attempted to determine the heat of reaction of ammonium nitrate dissolution in water. In order to determine how much heat a Thermos® calorimeter absorbed during a heat transfer, hot water and cold water were used in a calibration process. Following the determination of the Thermos® calorimeter's heat capacity, fresh water and ammonium nitrate were poured into the device, with the pre- and post-reaction temperatures recorded. Since ammonium nitrate absorbs heat from the surrounding water, a positive heat of reaction is to be predicted from its usage in cold packs. The heat of reaction was calculated using the temperature measurements and the Thermos® calorimeter's heat capacity; the result was 0.34 ± 0.2129 kJ/mol, which is not close to the literature value of 25.7 kJ/mol and suggests that the process was highly inaccurate and could be improved in several ways.