Ammonium Nitrate – Heat of Solution Lab
advertisement

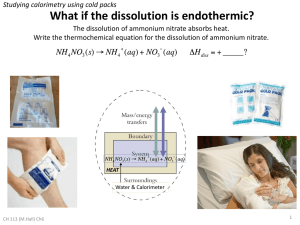
Ammonium Nitrate – Heat of Solution Lab Written for PULSE by Christopher Martin & Stephanie Nardei Editor: Stephanie Nardei Time: 1 class period Preparation 30 minutes Time: Materials: Heat of Solution of ammonium nitrate worksheet Ammonium nitrate Water Thermometers Graduated cylinders Styrofoam cups Abstract Students will measure the energy change caused by dissolving one mole of ammonium nitrate in water. Objectives Students will be able to: 1. Quantify the relationship between temperature, energy and heat 2. Understand that an endothermic reaction causes a decrease in temperature. National Science Education Standards National Science Standard The Physical Setting E. Energy Transformations Different energy levels are associated with different configurations of atoms and molecules. Some changes of configuration require an input of energy whereas others release energy. Arizona Science Standard Strand 5: Physical Science Concept 4: Chemical Reactions Investigate relationships between reactants and products in chemical reactions. PO 6. PO.10 Explain the energy transfers within chemical reactions using the law of conservation of energy. Concept 3: Conservation of energy and increase in disorder 1 PO.6 Distinguish between heat and temperature. Teacher Background Calorimeters are designed to be well-insulated, so no heat is gained from or lost to the surroundings. If no heating element is used to introduce heat in the system, the total heat (q) for the entire calorimeter system must equal zero. The total heat can be split into heats for each component in the system. Imagine a reaction in which solid ammonium nitrate (a component in some fertilizers and an explosive) is dissolved in water to produce an aqueous ammonium nitrate solution. NH4NO3 (s) NH4+ (aq) + NO3- (aq) The heat (qrxn) for this reaction is called the heat of solution for ammonium nitrate. When the reaction is finished, the system contains two substances, the calorimeter itself and the aqueous solution, and there is a heat associated with each component. The heat balance for this experiment is thus 0 = q = qrxn + qcal + qsoln The basic strategy in calorimetry is to use a temperature change and a heat capacity to determine a heat. In this experiment all substances start at the same initial and final temperatures. qcal = Ccal ΔT = Ccal ( Tf - Ti ) qsoln = Csoln ΔT = msoln ssoln ( Tf - Ti ) One typically determines the heat capacity of the aqueous solution (Csoln) from the mass of the solution (msoln) and the specific heat capacity of the solution (ssoln). The mass of the solution is the sum of the masses of the water and ammonium nitrate originally placed in the calorimeter. The specific heat capacity of the aqueous solution is usually close to that of pure water (4.184 J oC-1 g-1). The objective of this experiment is to determine the heat of reaction (in this case a heat of solution). The above equations can be combined and rearranged to yield a working equation: qrxn = - qcal - qsoln = - ( Ccal + msoln ssoln )( Tf - Ti ) Just as the heat capacity of a substance is an extensive property, so the heat of solution is an extensive property. It is generally more convenient to report intensive properties, thus the heat capacity of a substance is usually reported as a specific heat capacity, that is, the heat capacity per gram of substance. Similarly one can report a specific heat of solution, which is the heat a solution per gram of solute. More commonly, though, the molar heat of solution is reported. The molar enthalpy of solution (ΔHsoln) is the heat of solution (qrxn) per mole of solute (n). In this experiment the reaction is performed under conditions of constant pressure and the only work is "PV-work"; under these conditions the heat flow for the process equals the enthalpy change for the process. ΔHsoln = qrxn 2 n ABOVE INFORMATION TAKEN FROM: http://www.chm.davidson.edu/ChemistryApplets/calorimetry/HeatOFSolutionOfAmmoniumNitrate.html Related and Resource Websites Chemistry: The Science in Context http://www2.wwnorton.com/college/chemistry/gilbert/concepts/chapter13/ch13_1.htm David N. Blauch Calorimetry http://www.chm.davidson.edu/ChemistryApplets/calorimetry/HeatOFSolutionOfAmmoniumNitrate. html Brigham Young University Virtual ChemLab Community Heat of Solution Lesson http://pulse.pharmacy.arizona.edu/resources/heatofsolution.pdf Chemistry & Issues in the Environment (Ammonium Nitrate image taken from this website) http://www.elmhurst.edu/~chm/onlcourse/chm110/chm110.html Daniel High School Larry Jones Heat of Solution Reactions Lab http://www.sciencebyjones.com/heat_of_solutions.htm Ammonium nitrate on Wikipedia http://en.wikipedia.org/wiki/Ammonium_nitrate Wikimedia Commons two dimensional image of Ammonium Nitrate http://commons.wikimedia.org/wiki/Image:Ammonium-nitrate-2D.png Material Safety Data Sheet on Ammonium Nitrate http://www.jtbaker.com/msds/englishhtml/a6048.htm Answers.com on Ammonium Nitrate http://www.answers.com/topic/ammonium-nitrate The History of Ammonium Nitrate http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2001/Ammo niumNitrate/history.htm Activity 1. Have students use a graduated cylinder 10 and measure 100 ml of water. 2. Next, have them transfer the water to the styrofoam cup. 3. Record the temperature of the water. 4. Make certain they weigh accurately, between two and three grams of NH4NO3. 5. Next, they add the NH4NO3 to the water, continually swishing the cup gently, and with constant observation of the temperature until it remains constant for about 15-20 seconds. 6. Have them record this temperature. 7. After the students complete all data collection for this experiment, make sure they fill in the information into the Data Table below. 3 Data Table Mass of 75.0 mL of water Mass of NH4NO3 Initial temperature of water Final temperature of solution Temperature change Closure Have students answer the following questions so what they learned throughout the experiment can be synthesized. 1. Is the dissolution of the NH4NO3 in water an exothermic or endothermic process? 2. Assuming 1 mL of water has a mass of 1 gram and the specific heat of the dilute solution is the same as water, calculate the number of joules involved in the dissolution of the NH4NO3. (use actual mass of H2O) # of joules = 75 g x temperature change x 4.185 J/gºC 3. Record your answer: NH4NO3 ______________ J 4. How many moles of each substance were dissolved? # of moles = grams dissolved Molar mass NH4NO3 _____________ mole 5. Calculate the number of joules that would be involved if one mole of the substance were dissolved in water. # of J = mole # of J (see question 2 above) # of moles (see question 3 above) 6. If you have time, repeat the experiment with NaOH instead of NH 4NO3. You will need a new Data Table. 7. Compare the results of the two experiments. 8. Ask students questions about the experiments which demonstrate their comprehension of the material. Ask them for feedback. Embedded Assessment 4 Successful completion of the lab exercise and write up will demonstrate understanding. Homework If students did not finish the questions listed in the closure, you may have them do so for homework. 5