Planetary Atmospheres I - mo
advertisement

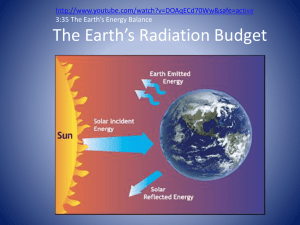
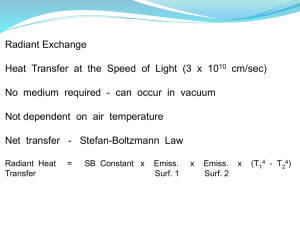
(Terrestrial) Planetary Atmospheres I Atmosphere: ◦ Layer of gas that surrounds a world Thin for terrestrial planets ◦ 2/3 of air within 10 km of Earth’s surface So what do atmospheres do? ◦ Pressure allows liquid phase of water ◦ Absorb and scatter light “Radiation shield” Ozone in Earth’s atmosphere absorbs UV radiation ◦ Wind and weather ◦ Can trap heat and warm the planet Molecules move fast and collide ◦ 500 m/s on Earth ◦ They therefore push on surfaces Aside: Why don’t they travel across a room that fast? Consider how gravity acts on a bunch of molecules in motion ◦ They “pile up” toward the surface ◦ The atmosphere below supports the atmosphere above Planets are able to hold onto their atmospheres longer if: ◦ They are large (stronger gravity) ◦ The temperature of the atmosphere is low Molecules don’t try as hard to escape Distance from the Sun Albedo: Reflectivity of surface and atmosphere Greenhouse Effect: Trapping a planet’s emitted radiation Sunlight rejected by planet ◦ Low Albedo: Darker: absorbs more Soil, trees, etc. ◦ High Albedo: Lighter: reflects more Cloud, ice caps, etc. If the sunlight is reflected, it can’t warm the planet Surface Typical Albedo Fresh Asphalt 0.04 Worn Asphalt 0.12 Coinifer Forest 0.09 to 0.15 Bare Soil 0.17 Green Grass 0.25 Desert Sand 0.40 Concrete 0.55 Fresh Snow 0.80 – 0.90 Different materials respond differently to different frequencies of light! Clouds reflect visible light. They do not reflect UV. Does albedo warm or cool a planet? Visible light from Sun absorbed by the ground Ground returns absorbed radiation as a continuous spectrum. ◦ Peaks in the infrared Greenhouse gases absorb these infrared photons ◦ Water Vapor ◦ CO2 (Carbon dioxide) ◦ CH4 (Methane) Keeps the lower atmosphere and ground warm ◦ Energy from the photons can be “exchanged” for kinetic energy through collisions Cloudy nights can be warmer than clear nights! Does the greenhouse effect warm or cool a planet? Be thankful for it… ◦ The infrared radiation emitted by Earth would escape straight back into space if not for the greenhouse effect. ◦ Earth would be at 3 oF if not for the greenhouse effect. ◦ We wouldn’t have liquid water. Venus has a high albedo and reflects 75% of incoming light. Why is it so hot (800 oF)? Do Mercury and the Moon have a greenhouse effect? Why or why not? Earth’s atmosphere is mostly diatomic nitrogen and oxygen (poor infrared absorbers). How would the temperature change if they were good infrared absorbers? Planetary climates are modeled as follows: ◦ Calculate Effective Temperature Assumes planet absorbs all radiation, emits freely ◦ Calculate Albedo Temperature Assumes that planet can reflect incident radiation ◦ Calculate Atmospheric Temperature Assumes atmosphere can inhibit radiation emission by the planet Variation of temperature with height Due to how atmospheric gas interacts with sunlight X-rays: ◦ Can remove electrons from atoms (Ionizes them) ◦ Can dissociate (break apart) molecules Ultraviolet: ◦ Can dissociate (break apart) molecules Visible light: ◦ Usually transmitted, sometimes scattered Infrared light: ◦ Absorbed by molecules ◦ Causes rotation and vibration in molecules X-rays (Page 304) Ultraviolet Visible Infrared The atmosphere scatters visible light ◦ Think in terms of light rays ◦ If no scattering, would see stars with Sun in view Blue light scattered more than red ◦ Red sunsets Troposphere gets the infrared light emitted by Earth ◦ Temperature drops farther from surface ◦ Has convection and storms Dense air Surface heat Infrared not significant here UV light absorbed by ozone here ◦ UV light from Sun ◦ The top layer absorbs more than the bottom ◦ Gets hotter with height to a point No convection, no weather Most gases absorb Xrays They get absorbed by the first dense gas they encounter ◦ Exosphere not dense enough This is the thermosphere Gets hotter higher up Very low density gas ◦ Faster molecules escape Boundary between atmosphere and space Gas very hot, but you wouldn’t feel it (low density)