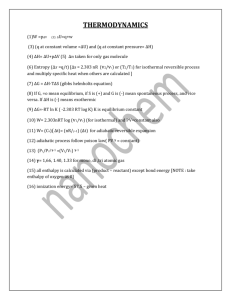
Thermodynamics Review
advertisement

ME 131B Fluid Mechanics
Solutions to Week One Problem Session:
Thermodynamics Review (1/12/98)
1. What is a thermodynamic property? What is the dierence between an intensive and
an extensive property? Give an example of both.
A thermodynamic property is a macroscopic characteristic which describes the
state of a system.
Extensive property depends on the size or extent of a system. Its value of an
overall system is the sum of its individual parts, like entropy and internal energy.
Intensive property is independent of the size of a system and is not additive, like
temperature and pressure.
2. What is a simple compressible substance? What does the state principle for a simple
compressible substance tell us?
Simple { there is only one reversible work mode which can alter the energy of the
system
Compressible { work mode is associated with volume change R p dV
The state principle states that two independent, intensive thermodynamic properties are sucient to fully determine the thermodynamic state of a simple compressible substance, like (T; v); (u; v). (Reminder: Pressure and temperature are
not independent of each other in the two-phase region.)
For a \non-simple" substance with n independent work modes, we need to know
a total of n + 1 independent, intensive thermodynamic properties to completely
specify its state.
3. What do the following laws of thermodynamics mean to you? Describe them in your
own words.
(a) Zeroth Law { Temperature
Equality in temperature is a necessary and sucient condition for thermal
equilibrium.
(b) First Law { Energy
Energy is conserved.
(c) Second Law { Entropy
1
Entropy can only be produced but not destroyed. (Be careful that it does not
mean that entropy of a system can never decrease. If we have enough heat
transfer out of a system, it is possible to have a decrease in the entropy of
the system.)
It is a powerful tool for us to determine the possible direction of a thermodynamic process.
4. Apart from the above laws of thermodynamics, what other basic principle(s) do we
usually apply in analyzing thermodynamic systems?
Conservation of mass
dmcv =
dt
X
m_ in
X
m_ out
Newton's law of motion
@ Z V~ ( dV ) + Z V~ V~ dA~ =
@t CV
CS
X
X
F~surface + F~body
5. What are the dierent transfer modes for
(a) Energy,
Mass transfer
Heat transfer
Work transfer
(b) Entropy?
Mass transfer
Heat transfer
There is no entropy transfer associated with work. This is a major dierence
between the two energy transfer modes, work and heat.
6. Write down the mathematical form of the First and Second Law for
(a) a close system,
X
X
U =
Qin
Wout
X Qin
S =
T + Ps
2
(b) an open system.
kinetic
z}|{
enthalpy
potential
2
z}|{
X
X
V
z}|{
dE = X Q_
_
h
+
W
+
m
_
(
+
gz )
out
in
dt
| {z }
| {z }
{z 2
}
|
heat trans. non-ow work
mass trans.
dS = X Q_ in + X ms
P_s
_ + |{z}
dt
T
{z
}
|
| {z }
heat trans. mass trans. production
Remarks:
Equation can be expressed in an overall or rate form.
Examples of non-ow work:
{ shaftR work,
{ any PdV type of work such as compression and expansion
Enthalpy consists of internal energy and ow work, h = u + P= (Hence, do not
double count the ow work in W_ out again!)
Internal energy is a measure of microscopic molecular activities while kinetic and
potential energies are measures of bulk uid motion.
7. A patent application describes a closed system which at steady-state conditions receives
a heat transfer of 500 W at a temperature of 400 K and develops a combined electrical
and mechanical power output of 500 W. There are no other energy transfers. Is this
claim thermodynamically feasible?
Since it is a closed system, there is no mass ow. Conservation of mass is trivial.
First law (conservation of energy) is satised.
Entropy production rate goes negative, hence, second law is violated.
As a conclusion, the claim is not thermodynamically feasible.
8. What is the ideal gas model? How about perfect gas model? Under what conditions
will these models be appropriate in describing real-life phenomena?
Ideal gas model:
{ It satises the thermal equation of state: p = R T where R is the gas
constant (dierent for dierent gases.)
{ u = u(T )
3
{ It is appropriate for high temperature and low pressure condition (negligible
interaction between participating gas molecules.)
Perfect gas model:
{ It is an ideal gas with constant specic heats Cp; Cv .
{ It is an appropriate model if the temperature variations between states are
not too large (together with the conditions for ideal gas behavior.)
9. What is the relation between the specic heats (Cp; Cv ) for an ideal gas?
According to denition
@h
Cp = @T
p
and
@u
Cv = @T
v
For ideal gases, they are reduced to ordinary derivatives
dh
du
Cp = dT
and
Cv = dT
Again, from denition
h = u + P = u + R T
(P = R T for an ideal gas)
Take derivative with respect to temperature of above equation, we have
Cp = Cv + R
Recall the denition of specic heat ratio
Cp = k
Cv
Solve the above two equations for Cp and Cv , we have
Cp = k k 1 R
and
Cv = k 1 1 R
10. What is an adiabatic process? When will it be realized physically?
Adiabatic process is a thermodynamic process in which no heat transfer occurs.
It is a good model if
{ System has good insulation.
4
{ Thermodynamic process proceeds at a much faster rate than heat transfer
does. For example, ow in a nozzle, valve.
11. What is an isentropic process? What is its signicance in thermodynamic analysis?
Isentropic process is reversible and adiabatic.
It serves as a limit for real adiabatic process.
12. What are some common causes for irreversibility in thermodynamic systems?
Dissipation like friction, viscous eects
Mixing
Spontaneous chemical reaction
Unrestrained expansion
13. Write down the Gibbs equation.
Tds = du
P
2
d
or
Tds = dh
dP
14. Derive the P T relationships for a perfect gas undergoing an isentropic process.
T1 k = constant
TP k k = constant
P k = constant
1
15. Sketch the following curves on a T s diagram
(a) constant pressure,
T
decreasing pressure
s
5
(b) constant density.
T
decreasing density
s
Based on the Gibbs equation, explain the dierence in the slope of the above two
curves.
The slope of any curve on the T s plane is characterized by the derivative dT=ds.
From the Gibbs equation,
Tds = dh dP
Tds = Cp dT dP
For a constant pressure process, dP = 0, we obtain
dT = T
ds
Cp
Hence, the slope of a constant pressure curve on a T s diagram is equal to T=Cp.
Similarly, we can obtain the slope of a constant density curve to be equal to T=Cv .
Since Cp = Cv + R, the constant density curve has a steeper slope than the
constant pressure curve at the same temperature.
6