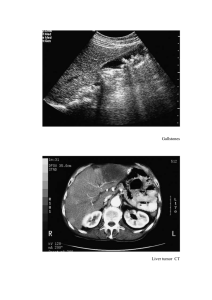
Taenia Species 1. Taenia saginata asiatica (Asian Tapeworm) Taxonomy Debate: Some consider this a distinct species, while others classify it as a subspecies of Taenia saginata based on DNA similarities. 2. Taenia multiceps (Multiceps multiceps) Rare Human Infection: It is a rare parasitic infection in humans, primarily affecting herbivorous animals. Morphology: Larvae (Coenurus): Morphological Features: Similar to T. saginata, but with key differences: Characterized by a unilocular cyst containing multiple scolices (hooks), which is why it is called Multiceps. Intermediate Host: Pig (as opposed to cow in T. saginata). Cysticerci Location: Primarily in the liver (instead of muscles). Scolex Features: Contains hooklets, which may be absent in mature worms. Size: Smaller, with 300-1,000 proglottids (segments). Geographic Distribution: Found mainly in Taiwan, Korea, China, Malaysia, Thailand. Not reported in India. Clinical Features: Clinical presentation, diagnosis, and treatment are similar to T. saginata. Diagnosis and Treatment: Similar to T. saginata, including stool examination for proglottids/eggs and possible imaging for cysticerci. Adult Worm: Length: 40-60 cm. Scolex: Pear-shaped with 4 suckers and an armed rostellum with 2 rows of hooklets. Eggs: 30 µm in size, similar to other Taenia species. Life Cycle: Definitive Host (where adult tapeworm lives): Dog, fox, and wolf. Intermediate Host (where larvae develop): Herbivores like sheep. Humans can serve as accidental intermediate hosts if they ingest contaminated food/water containing eggs. Oncospheres (eggs) hatch in the intestine, penetrate the walls, and migrate to various organs, especially the CNS (Central Nervous System), where they transform into the larval form (coenurus). Clinical Manifestations: The disease mainly affects herbivores’ CNS and is called gid, characterized by unstable gait and giddiness. In humans, space-occupying lesions in the CNS lead to: Headache Vomiting Paralysis Seizures Epidemiology: Occurs mainly in developing countries where dog populations are not controlled. Affected regions include: Africa: Uganda, Kenya, Ghana, South Africa The Americas: Brazil, Mexico, Canada, the United States India: A few cases reported, with a notable recent case of cerebral coenurosis from NIMHANS, Bangalore in 2011. Diagnosis: Based on gross and histological examination of the coenurus following surgical removal. Neuroimaging (CT or MRI) may also be used to visualize space-occupying lesions. Treatment: Surgical removal of the coenurus (larval cysts). Anti-parasitic medications may be used in certain cases, though surgery is typically the main approach. Key Points to Remember: T. saginata asiatica is primarily distinguished by its pig-based lifecycle and the location of cysticerci (liver vs. muscle in T. saginata). T. multiceps involves a complex lifecycle with dogs as definitive hosts and herbivores like sheep as intermediate hosts. Humans become infected accidentally, usually through ingestion of eggs from contaminated food or water. Both tapeworm species share similarities in diagnosis and treatment but have distinct epidemiological patterns and clinical manifestations. Echinococcus Species Echinococcus Overview: Echinococcus is a genus that causes hydatid disease in humans. Four species infect humans: E. granulosus – Causes cystic hydatid disease. E. multilocularis – Causes alveolar hydatid disease. E. vogeli and E. oligarthrus – Cause polycystic hydatid disease. Echinococcus granulosus (Cystic Hydatid Disease) History: Also known as dog tapeworm. Hydatid cysts were recognized as early as Hippocrates and Galen. Hartmann (1695) demonstrated the adult form in dogs. Goeze (1782) described the larval form (hydatid cyst). Structure: Head (Scolex): Shape: Pyriform (pear-shaped), about 300 µm in diameter. Features: 4 suckers and a rostellum (armed with two rows of hooklets). Neck: Short and thick. Strobila (Body): Composed of 3 proglottids (segments): Larval Form (Hydatid Cyst): 1. Immature segment 2. Mature segment 3. Gravid segment (broader than the others, filled with 100–1,500 eggs). Found in the liver and other viscera of humans and herbivores (e.g., sheep, cattle). The proglottids are similar to those of other cyclophyllidean cestodes. Habitat: Adult Worms: Reside in the intestine of dogs (the definitive host). Morphology of Echinococcus granulosus: Adult Worm: Size: 3–6 mm in length (much smaller than other cestodes). Eggs: Morphology: Similar to Taenia eggs. Oncosphere contains 6 hooklets, surrounded by an embryophore (protective outer layer). Larval Form (Hydatid Cyst): The hydatid cyst is the larval form of Echinococcus granulosus. Found primarily in the liver, lungs, or other viscera of intermediate hosts (humans, herbivores). Echinococcus granulosus: Life Cycle and Development Life Cycle of Echinococcus granulosus The life cycle of Echinococcus granulosus involves two hosts: Definitive Hosts (where adult worms live): Dogs and other canine animals (foxes, wolves). Intermediate Hosts (where larval forms develop): Eggs are ingested from contaminated food, water, or surfaces. 2. Transformation to Larva (Hydatid Cyst): After ingestion, the eggs release oncospheres (larvae) in the duodenum due to the rupture of the embryophore (outer shell). The oncospheres penetrate the intestinal wall and enter the portal circulation (bloodstream). 3. Migration to Organs: Herbivores such as sheep, cattle, and occasionally humans (humans are an accidental intermediate host). Oncospheres are carried by the bloodstream to the liver (60–70% of cases), lungs, or rarely other organs. Mode of Transmission: 4. Host Immune Response: Humans and other intermediate hosts acquire the infection by ingesting E. granulosus eggs found in dog feces (contaminated food, water, soil). Rarely, flies can serve as mechanical vectors, transferring eggs from feces to food or surfaces. The immune system attempts to destroy the larvae, causing an inflammatory response around the site of infection. While many oncospheres are destroyed, some escape destruction and develop into hydatid cysts. Development in Humans (Accidental Intermediate Host) The oncospheres are encysted by fibrous tissue (produced by fibroblasts), forming a fluid-filled cyst called a hydatid cyst. Cysts grow at a rate of 1 cm per month. 1. Ingestion of Eggs: 5. Formation of Hydatid Cyst: Full cyst development takes 10–18 months in sheep (intermediate host). 6. Maturation of Hydatid Cyst: Once mature, the hydatid cyst becomes infective to dogs and other definitive hosts. Humans are a dead-end host because dogs do not consume human organs. Development in Dogs (Definitive Host) 1. Infection by Consumption of Contaminated Viscera: Dogs or other canines acquire the infection by consuming the viscera (organs) of infected intermediate hosts (e.g., sheep) that contain mature hydatid cysts. 2. Transformation of Hydatid Cyst to Adult Worm: The hydatid cyst (larva) develops into an adult tapeworm in the dog's intestine. The adult worm matures and produces eggs, which are excreted in the dog’s feces. Key Points to Remember: Humans act as accidental intermediate hosts in the life cycle of Echinococcus granulosus. Transmission to humans occurs through ingestion of eggs from dog feces. The larval stage (hydatid cyst) forms in the liver, lungs, or other organs, leading to hydatid disease. Hydatid cysts grow slowly, maturing over 10–18 months. Dogs acquire infection by consuming the infected viscera of intermediate hosts and pass the eggs in their feces. Humans are dead-end hosts, meaning the cycle does not complete in humans. Echinococcus granulosus: Pathogenicity and Clinical Features Pathogenicity: The pathogenicity of Echinococcus granulosus is primarily due to the deposition and growth of hydatid cysts in various organs. Hydatid Cyst: Structure and Characteristics 3. Transmission to Other Hosts: Shape & Size: The eggs in the feces are infective to intermediate hosts (including humans), continuing the cycle. Fully developed hydatid cysts are unilocular (one chamber) and subspherical. Size: Varies from a few millimeters to more than 30 cm (common size 5-8 cm). Cyst Wall: Pericyst (Outer Layer): Host-derived fibrous tissue and blood vessels due to the host’s immune response. Ectocyst (Middle Layer): Parasite-derived tough, elastic, glycan-rich, acellular hyaline layer (approx. 1 mm thickness). Resembles the white of a hard-boiled egg. Endocyst (Inner Layer): Parasite-derived germinal layer (22-25 µm thickness). Forms the brood capsules and hydatid fluid. Produces protoscolices (future heads of tapeworms). Hydatid Fluid: Clear, colorless to pale yellow. pH: 6.7, Specific Gravity: 1.005–1.010. Contains sodium chloride, sodium sulfate, sodium phosphate, succinates. Antigenic, toxic, and anaphylactic. Brood Capsules and Protoscolices: Brood capsules are formed in the endocyst, containing protoscolices. Hydatid sand: Granular deposit formed by detached brood capsules and protoscolices. Types of Hydatid Cysts: Primary Cyst: Directly formed from the oncosphere after ingestion. Secondary Cysts: Result from the breakage of primary cysts due to trauma, leading to dissemination to other organs. Acephalocyst: Cysts lacking brood capsules and protoscolices. Endogenous Daughter Cysts: Formed when the brood capsule breaks within the cyst, producing smaller cysts inside. Fate of the Hydatid Cyst: Spontaneous Resolution: Some cysts may resolve on their own. Rupture: Can lead to: Formation of secondary cysts. Anaphylactic reaction due to leakage of hydatid fluid. Clinical Features of Hydatid Disease Age of Onset: Infection typically occurs in childhood but often manifests in adult life. Most Common Locations: Liver (60-70%, mainly the right lobe). Lung (20-30%). Rarely: Spleen, kidneys, brain, heart, bones. Asymptomatic Cases: Many cases are asymptomatic and only detected incidentally through imaging studies. Outcome and Disease Progression Symptoms: Symptoms primarily result from the pressure effects or complications caused by the cysts. Fever. Pressure Effects: Palpable abdominal mass. Hepatomegaly (enlarged liver). Abdominal tenderness. Portal hypertension and ascites (fluid accumulation in the abdomen). Obstruction: Daughter cysts may erode into organs like the biliary tree or bronchi, causing: Cholestasis (bile flow obstruction). Dyspnea (difficulty breathing). Secondary Bacterial Infection: May cause pyogenic abscess formation in hydatid cysts. Reactions: Cyst rupture can release hydatid fluid, leading to severe allergic reactions, including: Hypotension (low blood pressure). Syncope (fainting). Outcome depends on cyst size and location. Extrahepatic Cysts: More common in younger children, found in organs like lungs, brain, and orbit. Multiple Cysts: Found in 20-40% of cases, with involvement of multiple organs. Key Points to Remember: Echinococcus granulosus forms hydatid cysts, which grow slowly and are typically asymptomatic until large enough to cause organ compression. Liver and lung are the most common sites for cyst formation. Anaphylactic reactions can occur if cysts rupture. Multiple cysts or involvement of multiple organs is seen in a significant proportion of cases. Laboratory Diagnosis of Echinococcus granulosus Infection 1. Microscopic Examination of Hydatid Fluid: Procedure: o Aspirate hydatid fluid from cyst and examine under the microscope o o for brood capsules and protoscolices. Hydatid sand can be detected by placing a drop of centrifuged fluid between two slides and rubbing them together. The grating sensation indicates sand grains. Purulent material can be treated with hydrochloric acid for better visibility. Confirmatory Tests: 2. Histological Examination: Surgically removed cysts are examined for cyst wall layers and brood capsules using histopathological stains: o Giemsa stain o Hematoxylin and Eosin (H & E) o Periodic Acid-Schiff (PAS) stain These stains help demonstrate the three cyst wall layers (pericyst, ectocyst, endocyst) and attached brood capsules. 3. Antibody Detection: Screening Tests (60-90% Sensitivity): Indirect Hemagglutination (IHA) Latex Agglutination Test (LAT) Indirect Fluorescent Antibody (IFA) Test Enzyme-Linked Immunosorbent Assay (ELISA) Immunodiffusion and Electroimmunodiffusion: Detect antigen-5 (arc-5). Western Blot: Detects antibody against antigen B fragment (8-12 kDa band). 92% sensitivity, 100% specificity. Specific for IgG4 antibodies. o Antigen B is preferred for seroepidemiological studies. Additional Notes: Antibody tests can’t differentiate between recent and past infections. Liver cysts show better serological results than extrahepatic cysts. Alveolar echinococcosis shows better results than cystic echinococcosis. 4. Antigen Detection: Tests for detecting specific antigens in serum or urine: o ELISA, CIEP (Counter-current Immunoelectrophoresi s), LAT. These methods can differentiate between recent and past infections and serve as prognostic markers for treatment monitoring. 5. Imaging Methods: X-rays: Simple, inexpensive method to detect hepatomegaly and calcified cysts (can also detect cysts in lungs). Ultrasound (USG): Imaging method of choice due to low cost and high diagnostic accuracy (90%). Detects both single and multiple cystic lesions. Water lily sign: Fluid leakage causes germinal layer collapse, which floats in the cyst cavity. Daughter cysts: Appear as cysts within cysts, cartwheel or honeycomb appearance. Detects complications like biliary obstruction. Monitors treatment response and is used in epidemiological studies. Computed Tomography (CT): 90-100% sensitivity for cyst detection. More accurate for detecting number, location, and complications of cysts. Better than USG for detecting calcified cysts (calcification takes 5-10 years to develop). Superior for detecting extra-hepatic cysts and distinguishing from other cystic lesions. Prognostic marker: Can be used to monitor disease progression. Magnetic Resonance Imaging (MRI): Higher contrast resolution provides clearer images of cysts. Can be used as an alternative to CT scans but poor at detecting calcified cysts. 6. Molecular Methods: PCR targeting mitochondrial DNA has been developed for Echinococcus genotyping. PCR-RFLP (Restriction Fragment Length Polymorphism) can identify 10 genotypes (G1-G10). o o G1 genotype is the most common in India. Each genotype has a host specificity and geographical distribution. 7. Skin Test (Casoni Test): Immediate hypersensitivity reaction to hydatid fluid antigens. Developed by Casoni in 1911: o Procedure: Inject 0.2 mL of antigen into one arm and sterile saline o in the other arm as a control. Interpretation: A large wheal (≥5 cm) with pseudopodia forms at the test site within 30 minutes in sensitive patients. Limitations: Low sensitivity (60-80%). False positives due to cross-reaction with other cestodes. Obsolete: Largely replaced by serological tests. 8. Other Tests: Eosinophilia: Present in 20-25% of cases. Hypergammaglobulinemia: Common in patients with hydatid disease. Monitoring Treatment Response: Imaging methods (USG, CT, MRI): Monitor cyst size reduction or disappearance. Antigen detection: Antigen levels decrease as the patient improves. Key Points to Remember: Imaging (especially USG and CT) is essential for detecting cysts and monitoring progression. Antibody and antigen detection tests are valuable for diagnosis, but cannot differentiate between past and recent infections. Molecular methods (PCR) offer high specificity for identifying genotypes and understanding regional epidemiology. Casoni test is now obsolete due to low sensitivity and false positives. Treatment of Echinococcus granulosus Infection 1. Treatment Considerations: Decision-Making: Based on size, location, manifestations of cysts, and the overall health of the patient. 2. PAIR (Puncture, Aspiration, Injection, and Re-aspiration): Alternative to surgery. Steps involved: 1. Percutaneous puncture of the cyst. 2. Aspiration: Remove 10–15 mL of cyst fluid. 3. Injection of scolicidal agents (e.g., hypertonic saline, cetrimide, or ethanol). 4. Re-aspiration: Remove fluid after 5 minutes. Benefits of PAIR: Higher cure rate. Lower recurrence rate. Less complications and shorter hospitalization compared to surgery. Indications for PAIR: Larger hepatic cysts. Cysts within cysts. Multiple cysts. Cysts with detached membranes. Contraindications for PAIR: Superficially located cysts (risk of rupture). Inaccessible cysts or extrahepatic cysts. Cysts communicating with the biliary tree. 3. Surgery: Definitive treatment but should be reserved for: PAIR contraindicated or refractory cases. Secondary bacterial infections. Advanced disease. Disadvantages of Surgery: High recurrence rate (2–25%). Postoperative complications (10–25%). Preoperative Use of Albendazole: Reduces cyst size and prevents recurrence. Dosage: 15 mg/kg/day, divided into two doses. Given 4 days before to 4 weeks after surgery or PAIR. 4. Antiparasitic Agents: Albendazole: Drug of choice. Used to reduce cyst size before PAIR or surgery and to prevent recurrence. Administered for 4 days before and up to 4 weeks after the procedure. Alternative: Praziquantel (used when albendazole is not available or suitable). 5. Percutaneous Thermal Ablation: Non-invasive method. Involves radiofrequency ablation of the cyst's germinal layer. Offers an alternative to PAIR in certain cases. Key Points to Remember: PAIR offers a minimally invasive option with fewer complications and a higher cure rate than surgery. Surgery is the gold standard when PAIR is not suitable but carries higher risks of recurrence and complications. Albendazole is crucial for preoperative preparation and preventing recurrence, while praziquantel is an alternative. Percutaneous thermal ablation is a non-invasive option to consider for cyst management. Echinococcus multilocularis, Echinococcus oligarthrus, and Echinococcus vogeli: Laboratory Diagnosis:Imaging Methods: USG, CT Scan, and MRI: Detect cyst number, size, extent of the lesion, and calcification. Antibody Detection Tests: Echinococcus multilocularis (Alveolar Hydatid Disease) Morphology: Size: Smaller than E. granulosus (1.2 – 3.7 mm in length). Life Cycle: Hosts: Definitive Host: Foxes, wolves (also dogs and cats). Intermediate Host: Small wild rodents (e.g., squirrels, voles, mice). Human: Accidental intermediate host. ELISA: Sensitive (86-97%). Cross-reacts with E. granulosus (false positives). Western Blot (using Em-18 antigen): Highly sensitive (97%) and specific (100%). Does not cross-react with E. granulosus. Histopathology: PAS staining (Periodic Acid Schiff) after FNAC (Fine Needle Aspiration Cytology) or biopsy: Similar to E. granulosus life cycle, with differences in hosts. Clinical Features: Treatment and Prevention: Causative Agent of Alveolar (Multilocular) Hydatid Disease: o o Cysts have multiple locules (cavities). Cysts lack fluid or free brood capsules or scolices. Detects multiloculated cysts and necrosis. Similar to E. granulosus treatment: o o Surgery or PAIR (Puncture, Aspiration, Injection, and Re-aspiration). Albendazole for preoperative treatment and to prevent recurrence. Echinococcus oligarthrus & Echinococcus vogeli Hosts: Definitive Hosts: o o E. oligarthrus: Wild felids (wild cats, jaguars, pumas). E. vogeli: Wild brush dogs. Intermediate Hosts: Rodents (e.g., paca, spiny rats, opossums). Life Cycle: Only 80 cases of polycystic hydatid disease reported so far. Most cases are seen in humid tropical forest areas of Central and Northern South America: Similar to E. granulosus. Clinical Features: E. oligarthrus: Rare human infections. 4 cases reported: 3 involving the orbit and 1 involving the heart. E. vogeli: Most common in the liver (80%) followed by lungs and other organs. Symptoms are similar to cystic echinococcosis. Epidemiology: E. oligarthrus and E. vogeli are very rare in humans: Brazil, Colombia, Panama, Venezuela. Laboratory Diagnosis: Dependent on imaging, histopathology, serology, or molecular methods (e.g., PCR). Treatment and Prevention: Similar to E. granulosus treatment. Key Points to Remember: E. multilocularis causes alveolar hydatid disease with multiple locules and no brood capsules. It's diagnosed with Western blot using Em-18 antigen. E. oligarthrus and E. vogeli rarely infect humans and cause polycystic hydatid disease. Cases are mostly seen in tropical regions of South America. Diagnosis relies on imaging, serology, and histopathology. Treatment is similar to that of E. granulosus, including albendazole, PAIR, and surgery for more severe cases. Hymenolepis nana General Overview Common Name: Dwarf tapeworm. Smallest cestode infecting humans. Etymology of Hymenolepis Hymen: Thin membrane covering the eggs. Lepis: Covering. Nana: Small size. Discovery First detected by Bilharz in 1857 in the small intestine. Epidemiology Most common tapeworm infection globally, affecting 50-75 million people. Prevalence: 0-4%, with higher prevalence in children (up to 16%). Rostellum: Single row of 20-30 hooklets. Neck: Long and gives rise to proglottids. Strobila: Composed of 200 segments (proglottids). Mature proglottids: Contain both male and female reproductive organs. Genital pore: Opens laterally on the same side. Uterus: Lobulated wall. Testes: Three testicular follicles. Egg Size: 30-47 µm. Shape: Round to slightly oval. Structure: o Morphology Adult Worm Size: Small (1-4 cm). Resides in the upper two-thirds of the ileum. Structure: Consists of head, neck, and strobila. o o Head/Scolex: Globular shape. 4 suckers. Two membranes: Outer egg shell. Inner embryophore containing yolk granules. Oncosphere: Contains 6 hooklets. Polar filaments: 4-8 filaments emerging from thickened poles of the embryophore. Appearance: Non-bile stained (colorless in saline mount). Infective Form: Eggs. Larva Form: Cysticercoid. Structure: Solid, except for the proximal part, which is vesicular and contains the scolex. Key Points to Remember Mode of Transmission: 1. Ingestion of contaminated food and water with eggs. 2. Autoinfection: Ingestion of eggs released in the small intestine, leading to reinfection. H. nana is the smallest cestode and can be identified by its distinct egg morphology (non-bile stained, two membranes, polar filaments). The adult worm has a scolex with hooklets and a strobila with many proglottids. The egg is the infective and diagnostic form. Prevalence is high in children, with 50-75 million affected worldwide. Lifecycle Process: Hymenolepis nana: Life Cycle, Diagnosis, and Treatment High-Yield Notes Life Cycle of Hymenolepis nana Indirect Life Cycle Direct Life Cycle Hosts: Hosts: Definitive host: Humans. Intermediate hosts: Insects (e.g., rat fleas like Pulex irritans and Xenopsylla cheopis). Definitive host: Humans (only host). Other hosts: Rodents (rats and mice). Eggs hatch in the small intestine. Larvae penetrate the intestinal wall and develop into cysticercoid larvae in 4–5 days. The intestinal villi rupture, releasing the larvae into the gut lumen. Larvae mature into adult worms in 10–12 weeks. The adult worm undergoes fertilization and produces eggs. Eggs are passed in the feces, which are infective to humans. Autoinfection ensures persistence of infection, despite the worm's lifespan of only 4–10 weeks. Mode of Transmission: Rare: Humans acquire infection by ingesting fleas containing cysticercoid larvae. Lifecycle Process: Eggs are ingested by insects (fleas). Embryo hatches and penetrates the insect's intestine, developing into cysticercoid larvae. Infected fleas are consumed by humans, and the larvae develop into adult worms in the human intestine. The adult worm produces eggs, which are passed in the feces. Clinical Features and Pathogenicity Usually asymptomatic. Severe infections with 1000–2000 worms may cause: o o o o o Anorexia Abdominal pain Headache Dizziness Diarrhea Laboratory Diagnosis of H. nana Stool Examination: o Detect non-bile stained eggs with polar filaments between the shell membranes. Eosinophilia: May be present in some cases (≥5% eosinophils). Treatment Praziquantel: o o Dose: 25 mg/kg once. Effective against both adult worms and cysticercoid larvae in the intestine. Nitazoxanide: o o Dose: 500 mg twice a day for 3 days. Alternative treatment. Key Points to Remember Direct Cycle: No intermediate host; autoinfection is common, leading to persistent infection. Indirect Cycle: Fleas are the intermediate hosts, and human infection is rare. Diagnostic Clue: Non-bile stained eggs with polar filaments are characteristic. Treatment: Praziquantel is the drug of choice, with Nitazoxanide as an alternative. Hymenolepis diminuta and Dipylidium caninum: Hymenolepis diminuta (Rat Tapeworm) rostellum with 1–7 rows of hooklets. Life Cycle 1. Definitive host: Dogs and cats (humans are rarely infected). 2. Intermediate host: Insects (primarily fleas). Common Name: Rat tapeworm. Similarity to H. nana: Similar to H. nana, but with some differences (see table 10.6). Life Cycle: Indirect cycle only (unlike H. nana, which has both direct and indirect cycles). Intermediate host: Essential for the life cycle. Insects such as lepidopterans, myriapods, and beetles act as the intermediate hosts. Definitive host: Rodents, typically rats. Human Infection: Rare, with fewer than 500 reported cases (mainly in India, Japan, and Italy). Common Name: Double-pored tapeworm. Common Hosts: Dogs and cats (humans are rarely infected). Morphology o Resembles the indirect cycle of H. nana. Clinical Features Mostly Asymptomatic. Rare symptoms may include: o o o o o Indigestion Loss of appetite Diarrhea Pruritus ani (itching around the anus) Abdominal pain Children are more commonly affected. Laboratory Diagnosis Adult Worm: o Mode of Transmission: o Humans acquire the infection by ingesting fleas containing cysticercoid larvae. Life Cycle Similarity: o Dipylidium caninum (Double-Pored Tapeworm) Hosts: Length: 10–70 cm. Scolex: Contains four oval suckers and a Stool Examination: Eggs: 25–40 µm in size, typically in groups of 15 (called egg packets). Proglottids: Barrel-shaped (3.2 mm wide), resembling cucumber seeds when fresh and rice grains when dry. Proglottids contain two sets of reproductive organs with two genital pores, hence the name double-pored tapeworm. Treatment Praziquantel: Drug of choice for treatment. Key Points H. diminuta: Rat tapeworm, has an indirect cycle with insects as the intermediate host. Rare human infection. D. caninum: Double-pored tapeworm, commonly infects dogs and cats. Human infection occurs through ingestion of fleas containing cysticercoid larvae. Laboratory Diagnosis: Eggs in egg packets and barrel-shaped proglottids. Treatment: Praziquantel is the treatment of choice for both infections.