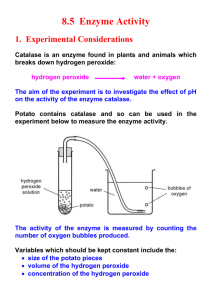
Yusuf 1 Catalyzing Change: Investigating How Environmental Variables Influence Catalase Efficiency Report By: Yusuf Ali Class: SBI4U0 Date: April 16, 202 Yusuf 1 INTRODUCTION: Proteins are the fundamental supports of all cellular functions; they have a very complex structure built at four levels. First, there is the linear amino acid sequence coupled with peptide bonds; the second structure built by hydrogen bonds involves alpha-helices and beta-sheets. The many side chain interactions taking place include hydrophobic interactions, ionic bonds, and disulfide bridges. The quaternary structure is a grouping together of several protein subunits, hence a similar interaction that will, in turn, stabilize this structure (see figure 1 in the Appendix for a diagrammatic representation). Among the myriad kinds of proteins in our bodies, there comes catalase. Proteins can denature, actually—it is the condition in which proteins lose their configuration for function due to factors such as temperature, pH changes, or other destabilizing agents acting on their stable noncovalent bonds. Though some denaturation processes are reversible and can regain functionality, most of them, under very harsh conditions, lead to a change in the irreversible state of protein functions. Catalase is critical in protecting cellular structures from intracellular poison by rapidly converting hydrogen into water and oxygen, as summarized in the catalase in Appendix Figure 2. Hydrogen peroxide represents a standard end product of metabolism and portends grave threats to one's health if a certain level of its accumulation gets to be tolerated in the body. Such accumulation might lead to the damage of DNA, proteins, and lipids (Rasheed). Catalase activity by its enzymatic action is required for the health and metabolic efficiency of all aerobic organisms since it avoids this accumulation. This reaction happens at the active site of the enzyme, in which its specific sequence of amino acids permits rapid conversion of hydrogen peroxide into less harmful molecules. This is in turn done to avoid possible damage or death of cells that would be occasioned by the accumulation of hydrogen peroxide in case the catalase enzyme malfunctions and its function is lost. PURPOSE: This lab exercise aimed to investigate different catalase activity factors, including enzyme concentration, substrate concentration, pH, and inhibitors. This lab will further try to understand how these factors make the catalase function differently in different Yusuf 2 physiologists. From the study, we shall further understand a sense of what enzymatic behavior means, and this will add more knowledge to our studies. HYPOTHESIS: Experiment A The hypothesis is that if we allow the reaction system to have more amounts of catalase-soaked disks, then it should produce a larger volume of oxygen gas and, therefore, yield an increment in water displacement inside the gas collection tube. The reasons behind this raise in formation can be well understood because additional catalase would present more active sites of the enzyme to speed up the reaction that gradually improved the rate at which the substrate hydrogen peroxide was decomposed into water and oxygen. Experiment B The hypothesis, in this case, explains that an additional amount of the substrate (hydrogen peroxide) will increase the rate of oxygen (O2) production. This increase in oxygen production is, therefore, likely if the observation that greater availability of hydrogen peroxide allows for more frequent catalytic reactions by the enzyme, enhancing product formation until the enzyme reaches its saturation point. Experiment C From the article "Bioactive Amines in Fresh Beef Liver and Influence of Refrigerated Storage and Pan-Roasting 2016," oxygen production will be at its maximum when pH ranges from 6.71-6.92. At this peak, it attains its structural and functional preferences. Any deviation from this optimal pH will reduce the activity because of the changes that occur in the enzyme's active site due to the breaking down of the hydrogen and ionic bonds within the protein structure. Experiment D Adding copper sulfate to the catalase-catalyzed reaction should reduce the rate of oxygen production. This would likely depress the reaction rate because copper ions can alter the conformation of the catalase and its activity, possibly occluding its active site where the substrate binds typically. This could interfere with the efficiency of the Yusuf 3 enzyme in catalyzing the breakdown of hydrogen peroxide. Yusuf 4 Experiment E The maximum expected oxygen production is from 36°C to 40°C, very close to the average body temperature of a cow, which is approximately 38°C (Ontario.Ca, 2007). The structure and kinetic properties of the enzyme would effectively align for catalysis at this temperature range. In other words, significantly deviating from this temperature—both higher and lower—will result in lessened enzyme activity and oxygen production due to thermal denaturation or lowered molecular mobility. MATERIALS AND PROCEDURE: absolute pressure sensors Materials: ● small square bottle ● ice ● rubber stopper ● silver nitrate ● 100 mL gas collection tube, ● copper sulfate ● a 10 ml graduated cylinder ● distilled water ● buret clamp holder ● hydrogen peroxide ● metal lasagna pan ● ● filter paper a variety of liver from different species (poultry, pork and beef) ● pH buffers of 2, 4, 6, 7, 8, 10, 12 ● beral pipettes ● hot plates ● hole puncher ● thermometers ● beef liver ● temperature probes ● stopwatch Work as a team (Lab ) at your laboratory table to perform the experiments. Each group should complete a portion of High - Tech and Low tech setups. All groups should complete part A. The teacher will assign one additional activity to each group from parts B, C, D, or E. Share data for B, C, D, & E with other groups. Each person should plot data on their own five graphs. Yusuf 5 OBSERVATIONS: Part A: Table 1: Data of Oxygen Generation Compared to Number of Discs over 5 Minutes Volume of Water Displaced (mL) Time(s) 4 Disks 3 Disks 2 Disks 1 Disk Distilled Water (Control) 0 0 0 0 0 0 30 10 15.8 20.5 12.3 0.1 60 25 19.8 31 16.8 0.1 90 30 22.8 41 22.8 0.1 120 35 24.8 47.5 27.3 0.1 150 36 25.8 51 31.3 0.2 180 37 27.8 52.5 35.3 0.2 210 37.5 28 53 38.8 0.2 240 37.7 28.3 53.5 40.8 0.2 270 38 28.3 53.7 43.3 0.2 300 38.2 28.3 53.7 45.8 0.2 Yusuf 6 Table 1.1: Details On the Production of O2 Gas to Varying Amounts of Catalase Disks Across 5 Minutes Number of Discs Qualitative Observations 1 catalase disks First, oxygen production is very high within 20 seconds, and then it slightly drops from that time. After around 75 seconds, it remained relatively stable in a linear trend until the end. 2 catalase disks Oxygen is quickly generated at the 50-second mark, followed by foaming up of the surface of the reaction vessel. This is then followed by an invariant decrease in the rate of oxygen production over the next minutes until it reaches a plateau. 3 catalase disks Oxygen is rapidly generated within 30 seconds, then it is followed with a decrease in the rate of oxygen production. 4 catalase disks Production of oxygen is fast for the first minute and then the speed of the reaction slowed as the reaction progressed and finally plateaued. 3 (Control) Very little to none oxygen gas was formed during this trial. Part B Table 2.0: Data of Oxygen production using Different Volumes of H2O2 over 3 Minutes Volume of Water Displaced (mL) Time (s) 6 mL of H2O2 4 mL of H2O2 3 mL of H2O2 2 mL of 1 mL of H2O2 H2O2 0 0 0 0 0 0 30 10 16 6.9 4.7 1.7 60 25 17 7.9 4.8 1.7 90 30 17 8.1 4.8 1.7 120 35 17 8.3 4.8 1.7 150 36 17.1 8.4 4.8 1.7 180 37 17.1 8.4 4.8 1.7 Table 2.1: Details On the Production of O2 Gas to Different Amounts of H2O2 Across 3 Minutes Yusuf 7 Amount of H2O2 (mL) Qualitative Observations 6 ml Oxygen production started rapidly and continued swiftly for the first minute before the reaction rate began to slow down and eventually reached a steady state.. 4 ml The formation of oxygen bubbles was initially vigorous at the start of the reaction and gradually tapered off after the first 30 seconds, with no further significant changes observed. 3 ml This condition followed a similar pattern to the 4 mL setup, where oxygen bubbles formed relatively quickly within the first 30 seconds, then the pace decreased for the remainder of the experiment. 2 ml Bubble formation was gradual from the beginning and continued at this reduced rate until the 30-second mark, after which no further bubbles were observed. 1 ml Oxygen was produced at a notably slow pace for the initial 30 seconds, after which oxygen production ceased, resulting in no additional bubble formation. Part C Table 3.0: Data of Oxygen Generation Using Different pH Levels over 3 Minutes Volume of Water Displaced (mL) Time(s) pH 2 (6 mL) pH 4 (6 mL) pH 6 (6 mL) pH 7 (6 mL) pH 8 (6 mL) pH 10 pH 12 (6 mL) (6 mL) 0 0 0 0 0 20 0 o 30 0.5 1.6 4.6 8.2 38 14.5 28.5 60 1 2 5.8 13 42 20 29 90 1.5 2.2 6.8 16 45 20 29 120 1.6 2.4 7.7 18 50 20 29.1 150 1.7 2.9 8.5 20 51 20 29.1 180 1.7 3 9.2 22 52 21 29.1 Table 3.1: detailed Production of O2 Gas to Different Levels of pH Across 3 Minutes pH Level Qualitative Observations Yusuf 8 2 The reaction proceeded at a notably slow pace, and a few air bubbles escaped. Some bubbles were observed sticking to the sides of the reaction vessel. 4 There was foaming at the water's surface within the buret. 6 A thicker layer of foam along with a small number of bubbles was present on the water's surface in the buret. 7 Abundant foam formation occurred within the vessel during the reaction. 8 The reaction rate was significantly faster than in other trials, resulting in large variations within the data. 10 The reaction proceeded rapidly; however, there were no foamy bubbles, with air bubbles visibly rising from the bottom to the top of the reaction vessel. 12 The reaction was very vigorous, marked by the absence of foamy bubbles, with air bubbles ascending from the base to the top of the reaction vessel. Part D Table 4.0: Data of Water Displacement Using Different CuSO4 Concentration. over 3 Minutes Volume of Water Displaced (mL) Time(s) CuSO4 (1 M) CuSO4 (0.1 M) CuSO4 (0.05 M) 0 25 0 14 30 26 7 20 60 26.5 9 24 90 26.8 11 26 120 27.3 13 27 150 27.5 14 28 180 27.7 15.5 28 Table 4.1: Details On the Production of O2 Gas to Different Concentrations of CuSO4 Across 3 Yusuf 9 Minutes Concentration of CuSO4 (M) Qualitative Observations 1M bubbling + foam + little bubbles present + very slow 0.1 M foam and little bubbles present + very slow 0.05 M bubbling + foam + little bubbles present + very slow Part E Table 5.0:Data of Water Displacement Over an Increase in Temperature over 3 Minutes Volume of Water Displaced (mL) Time(s) 0 - 5 °C 6 - 15°C 16 - 25°C 26 - 35°C 36 - 45°C 46 - 55°C 0 0 20.4 0 24 0 0 30 21.8 26.2 13.4 33 6.4 13 60 23.8 28.4 17.2 36 11,2 15.6 90 25.8 30 19 37 15 18 120 26.8 31 19.6 41 19.4 19 150 27.8 32.1 20 44 24.6 20.2 180 30.4 33.2 20.4 46 29.6 21 Table 5.1: Details On the Production of O2 Gas to Varying Temperature Ranges Across 3 Minutes Temperatures (℃) Qualitative Observations Yusuf 10 0 - 5 °C This temperature range was characterized by a rapid and dense formation of bubbles and foam within the reaction vessel, significantly obscuring visibility within. Foam accumulated on the water's surface within the vessel, covering approximately 6 mL of volume. Additionally, bubbling and foaming occurred within the water bath. After 1 minute and 30 seconds, the rate of bubble and foam formation notably declined for the remainder of the 3-minute reaction period. 6 - 15°C Bubbles were observed throughout the reaction, but their frequency diminished progressively as the reaction advanced. 16 - 25°C Initially, a considerable number of bubbles formed; however, as the reaction progressed, there was a noticeable decrease in the number of gas bubbles forming. 26 - 35°C A substantial quantity of bubbles was observed along the gas-water interface. 36 - 45°C Bubbles started to form and slight foaming was observed at the water's surface in both the water bath and the reaction vessel. The bubbles ascended from the base to the surface of the reaction vessel, with some clinging to the inner walls throughout the reaction. Bubble formation occurred at the bottom area near the reaction vessel. Foam-like bubbles formed at the water surface of the reaction vessel, while air bubbles from the bottom rose to the top surface of the vessel, with many accumulating along the inner side. 46 - 55°C ANALYSIS: Experiment A Graph #1 Oxygen Release by Catalase at Different Enzyme Concentrations Yusuf 11 Table 1 and Graph 1 both support the statement that the activity of catalase is directly proportional to the disks added. The two-disk setup had displaced a higher amount of water after 300 seconds, approximating 53.7mL, indicating increased activity and maximum production of oxygen. Interestingly, the activity observed with four disks, although much more significant than that with the control, actually amounted to less than that observed with two disks. This suggests that probably there exists a limitation in the availability of either the substrate or other factors affecting the efficiency of the enzyme at high concentrations. Yusuf 12 Experiment THEORY A In experiment A, the influence of various concentrations of catalase on the activity of the enzyme in promoting the decomposition of hydrogen peroxide was examined. According to the Kinetic Molecular Theory (KMT), the reaction rate, in general, depends on the frequency of effective collisions of the reaction particles. This theoretically implies that an increase in the concentration of the enzyme catalase increases the number of active sites that hydrogen peroxide has to attach to. Hence, the chance of effective collisions is also brought up. Consequently, it leads to an increased reaction rate. ("13.1.1: Effects of Temperature, Concentration, and Catalysts on Reaction Rates"). The latter was manifest in an increase in the rates of reactions that correlated with the number of discs soaked with the catalase enzyme. This is evident from the increased amount of oxygen produced as indicated through increased water displacement in graph 1 for the highest enzyme concentration (2 disks) as compared to a lower concentration of the same (1 disk). This shows that due to more collisions taking place, there is more catalase available. The 4-disk setup, however, did not give the highest oxygen production and hence did not conform to the simple prediction based on KMT. This difference could be attributed to errors during the experiments, for example, contamination. In addition, other factors that would slow down the reaction rate could be a limited amount of the hydrogen peroxide substrate available and perhaps interference amongst enzyme molecules, to such an extent at very high concentrations, that might reduce the efficiency of the active sites of the catalase molecules. Yusuf 13 Experiment B Graph #2 : Effect of Substrate Volume on Catalase Activity Measured by Oxygen Displacement As shown in Graph 2, the reaction that starts with 4 mL of hydrogen peroxide will evolve oxygen at the most significant rate initially. Still, the reaction that begins with 6 mL of hydrogen peroxide will produce the most total oxygen. The reactions with 2 mL, 1 mL, and 3 mL of hydrogen peroxide quickly level off, suggesting the reaction rate becomes limiting. Broadly, from these results, it can be said that with the quantity of oxygen produced, the volume of hydrogen peroxide used is proportionate; the maximum amount of oxygen is evolved with 6 mL. Experiment THEORY B In experiment B of this lab, the enzyme-substrate complex theory suggests enzymes are most effective in balanced concentrations of substrate and enzyme molecules. We tested the effect on catalase activity by varying substrate concentration. And the higher substrate concentration tends to take up even more sites of the enzyme's active sites until it gets saturated with the participation of all the active sites being catalyzed (Khan Academy, 2021). This is reflected in the qualitative and quantitative data of Tables 2.1 and 2.0. The rate of oxygen production was high; first, 6 mL of hydrogen peroxide would indicate a sufficient number of substrate molecules were available for the Yusuf 14 catalase active sites, favoring a high rate of catalytic reactions. This burst of activity occurs rapidly over approximately one minute and after that continues on a plateau. This indicates that the enzyme active sites either are possibly fully occupied or, indeed, the product removal and other factors could not allow any further increase in reaction rates. In the 4 mL of hydrogen peroxide condition, there is a high production of oxygen in the beginning that is reduced most likely because it experiences smaller substrate molecules than in the 6 mL case and therefore decreases enzyme-substrate complex formation as time goes on for the experiment. This reduction starts to be manifested after 30 seconds when the available substrate begins to fall below the amount of enzyme. One can, therefore, use an analogy that the body metabolizes a smaller meal in a way that enzymes will, without doubt, catalyze the processes of available substrates without being at the point of overload. Experiment C Graph #3: Impact of pH on Catalase-Driven Oxygen Production Over Time From the findings of Graph 3 and Table 3.0, catalase activity is demonstrated to be affected by pH levels. Where 32 mL displaced water over 180 seconds, the most significant activity of catalase, allowing for the highest amount of oxygen production, Yusuf 15 was expressed at pH 8. Catalase also shows marked activity at pH 12, almost the same as that at pH 7, indicating some tolerance for alkaline conditions. On the other hand, the enzyme was poorly active under acidic conditions, in which the lowest pH was registered at 2. The pattern of activity across the range of pH values was standard for enzymes, with maximum activity in or around neutral to slightly primary conditions and an abrupt decrease in more acidic settings. Experiment C It further investigated in experiment C how pH affects catalase, which is one of the outstanding examples in the study of enzyme structure-function relationships. The data regarding the effect of the respective pH of the media on the catalase activity in converting hydrogen peroxide to oxygen are given in Tables 3.0 and 3.1. Enzymes are proteins; they are formed of amino acids. It presents side chains with some that can either accept or donate protons, and fluctuations in pH can influence the side chains. However, the overall form and, especially at the active site, the enzyme's reactivity. Very high levels of either acidity or alkalinity in both could denature the structure of the enzyme that is three-dimensional and needed for binding with the substrate. This is an experiment that indicated various activity levels under different pH conditions. Enzyme activity significantly decreased at pH 2 and 4, where it was highly acidic, as evidenced by the slow reaction rate and minimal water displacement, if not a possible denaturation of the enzyme or distortion of the active site. At pH 6, though it had raised activity, it did not have the same level of activity that would be present for a neutral pH; this supports the concept that an enzyme's activity can be affected even by a slight deviation from the optimum pH. The highest catalase activity was exhibited at a pH of 8, where the reaction was noted to be "extremely fast," and maximal water displacement was experienced. This has ideally been suggested to provide the perfect environment for catalase activity, whereby the active site does not change from its optimal structure for the proper processing of substrates. On the contrary, at an alkaline pH of 10 and 12, the activity was still considerable. The fact that there were no foamy bubbles and the rate was very fast clearly stated that the active site was possibly in action but pointed to a likelihood that the overall shape of the enzyme was starting to be denatured under the alkaline conditions. Yusuf 16 Experiment D Graph #4: Catalase Activity in the Presence of Varying Concentrations of Copper Sulfate Graph 4 and Table 4.0 indicate that the oxygen gas production rate by catalase decreases with increasing concentration of copper sulfate. The highest concentration tested (1 M) obtained a significantly reduced amount of oxygen produced, amounting to about 2.7 mL, and this clearly showed high inhibition of the activity of the enzyme. At the lowest concentration of 0.05 M, on the other hand, significantly more oxygen, about 14 mL, was produced, and this indicated less inhibition. These, too, had vastly differing rates of reaction. The 1M reaction of copper sulfate was, however, much slower in comparison to the 0.05 M reaction of copper sulfate, which had higher initial rates that fell over time. This pattern would suggest that lower concentrations of copper sulfate increase activity and higher concentrations decrease it. Experiment D Experiment D of the paper set out to identify how different concentrations of copper sulfate (CuSO4) influenced the catalytic conduct of catalase. In the context of this factor (temperature), it was identified by both qualitative insights (refer to Table 4.1) and quantitative metrics (refer to Table 4.0). Under normal, the catalase efficiently Yusuf 17 decomposes hydrogen peroxide into water and oxygen, which during experiments is quantified in the measurement of oxygen gas formed. In the average advance of this reaction, it is rapid and vigorous, as seen from an intense oxygen production. However, adding some metal salts, including CuSO4, would significantly interfere with such a process. This demonstrates the concentration of CuSO4 at 1M, whereby the qualitative and quantitative data both show drastically reduced activity of the catalase, characterized by slow and scanty bubbling and foam, with minimal dislocation of the water. This could, therefore, mean that at high concentrations, copper ions are binding to the catalase, changing its structure, and consequently, its function is affected. When the CuSO4 concentration is dropped to 0.1M, the activity of the enzyme is still suppressed but this time with a slight increase in the volume of oxygen released over time. In lower concentrations, the CuSO4 concentrations from the 1 M would consequently mean that there is less copper ion concentration than the 1 M; therefore, less disturbance in the active site of the enzyme, hence probably some may recover for catalase activity. Catalase was, however, far less inhibited by the minimum concentration of 0.05 M CuSO4. Water displacement also approached normal levels. The fewer copper ions at this concentration are likely to have less of an effect on the structure of the enzyme and, hence, allow a more normal breakdown rate of hydrogen peroxide. Yusuf 18 Experiment E Graph #5: Temperature Effects on Oxygen Gas Production by Catalase The graph outlines the reaction of catalase with respect to temperature variations. Catalase has the best efficiency for its catalytic activities within a moderated temperature range of between 26°C and 35°C, often giving off the highest amount of oxygen. As temperatures decreased to 6–15°C, a much slower reaction speed was noted, whereby the enzyme activity is heavily curtailed under such excellent conditions. At each level of temperature, the reaction rate would change suddenly and critically at about 30 seconds into the experiment, only to stabilize shortly after in the optimal range. This is attributed to the fact that the substrate is fast reaching its exhaustion. Experiment E In experiment E, we tested for the effect of temperature on enzyme catalase structure and function. On analyzing the results presented in Table 5.0 and qualitative data from Table 5.1, it would appear that there is support for the molecular theory that Yusuf 19 temperature has a vital role concerning enzymes and their activity. Rising temperature can speed up molecular movement due to increased kinetic energy; it usually increases enzyme activity since the molecular movement becomes more vigorous and the collisions are more frequent and vigorous between the enzyme and the substrate molecules. This is clearly indicated from the data on the reaction rates and corresponding oxygen output, in which at high temperatures of 26°C - 35°C, there exists a vigorous reaction and corresponding high oxygen output. This explains that the conformation of an enzyme is perfectly arranged for the activities at these temperatures. During this range, there is the body temperature of an ordinary cow, which is 38°C (Ontario.Ca, 2007). The deactivation of most enzymes starts to occur at higher temperatures, in particular, within a range of 46°C to 55°C. This is mainly because the high temperatures lead to the denaturation of the protein structure of catalase, whereby high temperatures break stabilizing forces of importance, such as hydrogen and ionic bonds, and hydrophobic interaction within the tertiary and quaternary structures of the enzyme. This leads to disrupting the particular threedimensional conformation of the enzyme, including that of the active site, which is vital for the enzymes' catalytic activity, as explained in class. Interpretation: POSSIBLE SOURCES OF ERROR: The catalase lab experiment is thus susceptible to equipment limitations prone to introducing errors. Contamination is therefore the main vulnerability. Even the smallest impurities may show a marked effect on the enzymatic activity of the given source. This can lead to errors of judgment concerning the functionality of catalase. However, even though every care was taken to maintain the environment and the equipment in sterility, Yusuf 20 it cannot be ascertained that the place was completely free from contaminants, also, the apparatus used in the experiment itself, right from the clamps for holding the tubes to collect gases, could influence the measurements themselves. Another problem is with the foam formation; it may stick to the inner sides of the collection tube or float on the surface of the water. Thus an accurate reading of the displaced volume of water is not made. Another source of error that may be possible would be the loss of oxygen gas during the experiment. Even a small leak in the bottle top or while transferring the mixture would cause the oxygen gas to be lost, hence biasing the data. There were a few critical limitations with the use of the equipment. Precision in measurement greatly depended on the accuracy of the instrument used. For instance, a poorly calibrated thermometer would give wrong measurements for actual temperatures. Besides, variations in glassware used to measure solutions could have an impact on substrate concentrations, leading to systematic differences affecting the reaction rates. Yusuf 21 REVISITING THE HYPOTHESIS: Experiment A The hypothesis for Experiment A predicted that increasing the number of catalasesoaked disks would lead to a greater production of oxygen gas. The results partly confirmed this hypothesis. While increasing the disks from one to two did result in heightened oxygen output, adding three and four disks did not yield a corresponding increase in oxygen gas volume. This shows that there are additional limiting factors at play that disrupt the direct correlation between enzyme concentration and product output. Experiment B According to Experiment B, increasing the amount of the substrate (hydrogen peroxide) was expected to boost oxygen production until the point of enzyme saturation. The findings supported this hypothesis, showing clearly that the level of oxygen output increased along with the increasing amount of hydrogen peroxide. Experiment C Experiment C, as stated in "Bioactive Amines in Fresh Beef Liver and Influence of Refrigerated Storage and Pan-Roasting, 2016," suggested that the optimal pH range for catalase would be around 6.71-6.92. However, the data showed the highest oxygen production at pH 8, indicating a preference for a slightly more alkaline optimal pH for catalase in this experiment. The results also confirmed that deviations from this optimal pH would decrease catalase activity. Yusuf 22 Experiment D The hypothesis for Experiment D proposed that the introduction of copper sulfate would decrease oxygen production. The results supported this hypothesis, showing that copper ions inhibit catalase activity as anticipated. The data demonstrated an inverse relationship between the concentration of copper sulfate and catalase activity, supporting the notion that copper ions can inhibit the enzyme by altering its structure or blocking the active site. Experiment E The hypothesis for Experiment E expected the highest catalase activity at temperatures between 36°C and 40°C, aligning with the normal body temperature of cows. However, the highest oxygen production occurred at a slightly cooler range of 26°C - 35°C. Although this range is close to the predicted temperatures, it suggests that the optimal temperature for catalase activity might be slightly lower than the body temperature of cows. EVALUATION OF PROCEDURE: The experimental method is realistic and efficient, for it has replication redoable quickly, and its taking place is done quickly. This makes it user-friendly, given that the apparatus used during this experiment is easily set up, and the procedural instructions are easily read through, hence accessible even to people with little knowledge of science. A pump system could be added with facilities to inject hydrogen peroxide directly into the reaction vessel. This variation would help reduce the loss of oxygen in Yusuf 23 the process of manual transfer from the reaction vessel to the gas collection tube, as it would permit immediate direct placement under the gas collection tube and, therefore, save further the produced gas. STRENGTHS: The experiments are repeatable and, consequently, serve as a critical condition in the result and hypothesis verification of any scientific research; necessary materials are, moreover, easily searchable. WEAKNESSES: It may lead to deficiencies in the precision required by exact measurements, leading to inaccuracies in the volume of oxygen produced per reaction due to the application of essential laboratory equipment. LAB CONCLUSION: In conclusion, the laboratory experiment shows that the effects of enzyme concentration, substrate concentration, temperature, and pH on the inhibitors of catalase activity, such as copper sulfate, give the expected outcome. The obtained results confirm basic ideas of enzyme kinetics: that under some conditions, catalase activity reaches the highest point, while under conditions not maintained, that activity falls off. This includes saturation effects at high concentrations and optimal activity achieved at a specific pH level and temperature. APPENDIX: Yusuf 24 Figure 1: Four Levels of Proteins. Yusuf 25 Figure 2: The chemical reaction of hydrogen peroxide being catalyzed by catalase to produce water + oxygen REFERENCE BIBLIOGRAPHY: “Bioactive Amines in Fresh Beef Liver and Influence of Refrigerated Storage and PanRoasting.” Food Control, vol. 60, 1 Feb. 2016, pp. 151–157, www.sciencedirect.com/science/article/pii/S0956713515301250, https://doi.org/10.1016/j.foodcont.2015.07.037. “Four Levels of Protein Structure.” Www.mun.ca, 2010, www.mun.ca/biology/scarr/iGen3_06-04.html. Yusuf 26 Khan Academy. “Enzymes Review.” Khan Academy, 2021, www.khanacademy.org/science/ap-biology/cellular-energetics/environment al- impacts-on-enzyme-function/a/hs-enzymes-review. KHAN ACADEMY. (2022). Enzyme regulation . Khan Academy. https://www.khanacademy.org/science/ap-biology/cellular-energetics/environmentalimpa cts-on-enzyme-function/a/enzyme-regulation Ontario.Ca. “Cold Stress in Cows | Ontario.ca.” Www.ontario.ca, Jan. 2007, www.ontario.ca/page/cold-stress-cows#:~:text=Like%20all%20mammals%2 C%20cows%20are. Rasheed, Zafar. “Therapeutic Potentials of Catalase: Mechanisms, Applications, and Future Perspectives.” International Journal of Health Sciences, vol. 18, no. 2, 2024, pp. 1–6, www.ncbi.nlm.nih.gov/pmc/articles/PMC10915913/. “13.1.1: Effects of Temperature, Concentration, and Catalysts on Reaction Rates.” Chemistry LibreTexts, 17 July 2023, chem.libretexts.org/Courses/Fresno_City_College/Introductory_Chemistry_A toms_First_for_FCC/13%3A_Chemical_Equilibrium/13.1%3A_Collision_Theo ry_and_Reaction_Rates/13.1.1%3A_Effects_of_Temperature%2C_Concentrati on%2C_and_Catalysts_on_Reaction_Rates.