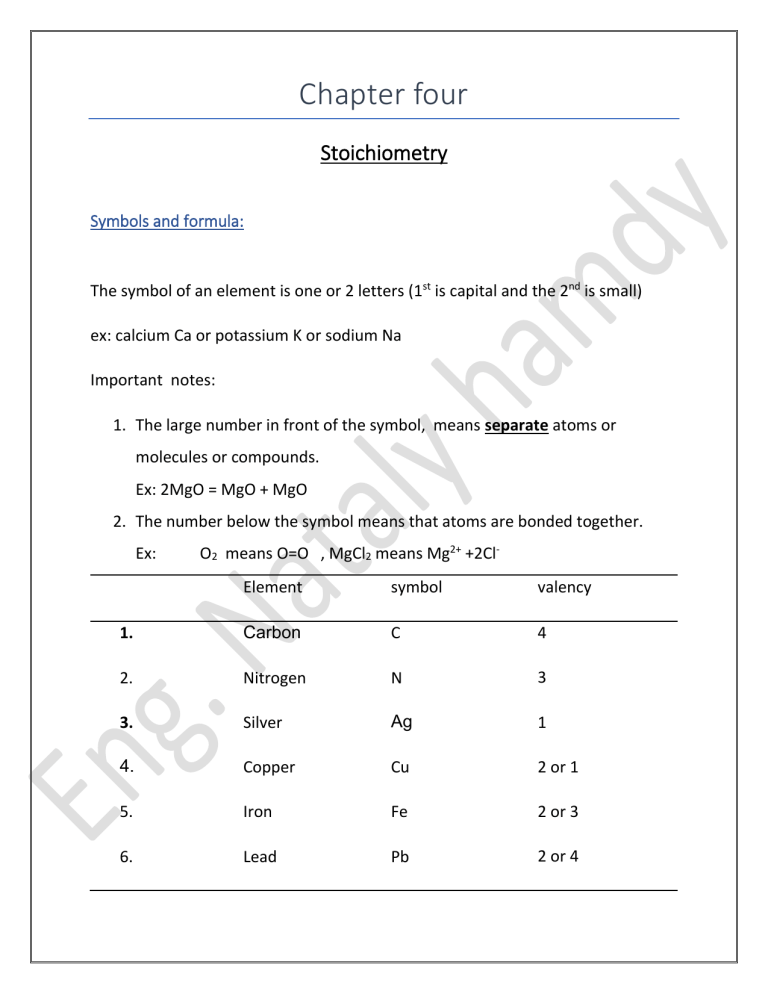
Chapter four Stoichiometry Symbols and formula: The symbol of an element is one or 2 letters (1st is capital and the 2nd is small) ex: calcium Ca or potassium K or sodium Na Important notes: 1. The large number in front of the symbol, means separate atoms or molecules or compounds. Ex: 2MgO = MgO + MgO 2. The number below the symbol means that atoms are bonded together. Ex: O2 means O=O , MgCl2 means Mg2+ +2ClElement symbol valency 1. Carbon C 4 2. Nitrogen N 3 3. Silver Ag 1 4. Copper Cu 2 or 1 5. Iron Fe 2 or 3 6. Lead Pb 2 or 4 7. Zinc Zn 2 8. Potassium K 1 9. Calcium Ca 2 10. Oxygen O 2 11. Sulfur S 2 For group of atoms having charge ( radicals): Charge Radical Formula +1 ▪ Ammonium ▪ NH4+ -1 ▪ Hydroxide • OH- ▪ Nitrate • NO3- ▪ Nitrite • NO2- ▪ Hydrogen • HCO3- carbonate -2 3- ▪ Hydrogen sulfate • HSO4- ➢ Sulfate ➢ SO42- ➢ Sulfite ➢ SO32- ➢ Carbonate ➢ CO32- ✓ Phosphate ✓ PO43- ✓ Phosphite ✓ PO33- Naming compounds: 1. ide: compound containing 2 different elements only (except ~~~~~~~~``~ hydroxide) ex: calcium chloride CaCl2 or potassium iodide KI 2. ~~~~~~~ate : compound containing plenty of oxygen ex: potassium sulfate K2SO4 3. ~~~~~~~ite : compound containing one oxygen less than ~~~~ate ex: potassium sulfite K2SO3 Working out the formula of a compound: 1. Write the symbols of the element/ radical with their valency. 2. Divide by the common denominator if present. 3. Cris-cross Ex: write the formula of : i. ii. Magnesium oxide: common number is 2 so by 2 Mg 2 O2 Mg 2/2 O2 /2 The symbol is MgO Potassium sulfate: K1 SO42- cris-cross K2SO4 iii. Iron (II)chloride: Fe 2 Cl1 cris-cross FeCl2 iv. Copper (II)iodide: Cu2 I1 CuI2 v. Copper(I) iodide: cris-cross Cu1 I1 cris-cross CuI ❖ Note: If a radical is involved and a number is to be put after it , a bracket is put around the radical. Examples: • Aluminium sulfate: Al3+ SO42- cris-cross Al2(SO4)3 • Ammonium phosphate: NH41+ PO43- (NH4)3PO4 ➢ Naming formula: ▪ FeO: iron(II)oxide ▪ NaNO3: sodium nitrate ▪ FeSO4: iron(II)sulphate ▪ ZnCO3: zinc carbonate ▪ Al(NO3)3: aluminium nitrate ▪ (NH4)2 SO4: Ammonium sulfate ▪ Na2S: sodium sulfide ▪ Mg3P2: magnesium phosphide ➢ Learn the following formula: ▪ H2O water ▪ HCl (aq) hydrochloric acid ▪ HCl(g) hydrogen chloride ▪ H2SO4(aq) sulfuric acid ▪ NO2(g) nitrogen dioxide ▪ NO(g) nitrogen monoxide ▪ HNO3(aq) nitric acid ▪ CO2(g) carbon dioxide ▪ CO (g) carbon monoxide ▪ SO3 sulfur trioxide ▪ SO2(g) sulfur dioxide ▪ H2O2(l) hydrogen peroxide ▪ NH3(g) ammonia gas ▪ NH4OH(aq) aqueous ammonia Note: Most of the common gases are diatomic molecules, ex: Cl2 , F2 , O2 , N2 ,H2 also Br2(l) and I2(s). Balancing equations: 1. Write the formulae of each substance in the word equation. Ex: Iron + hydrochloric acid → iron (II ) chloride + hydrogen gas Fe 2. + HCl → FeCl2 + H2 Check that the number of atoms of each element on R.H.S equals the number of atoms on the L.H.S. L.H.S R.H.S Fe : 1 1 H: 1 *2 2 Cl: 1 *2 2 we must put 2 in front of HCl (multiply by 2). Very important note: Never alter the number within a formula. Example: Lead(IV) oxide Pb4/2 O2/2 → lead(II) oxide + oxygen Pb2/2O2/2 + O2 → PbO PbO2 + O2 Then we balance each element in the equation. L.H.S R.H.S Pb: 1 1 O: 2 3 The PbO is causing the odd no. in R.H.S , SO multiply it by 2 the balance the equation. 2PbO2 • → 2 PbO + O2 State symbols: S → solid , l→ liquid , aq →aqueous dissolved in water/ solution in water. Ex: Na2CO3(s) + 2HCl(aq) → 2 NaCl(aq) + H2O(l) +CO2(g) Deduce the formula of compound from a model: