SCH4C1-02 Stoichiometry Test
advertisement

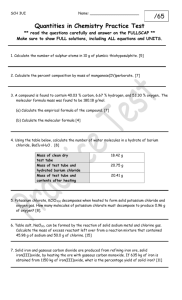
SCH4C1-02 Stoichiometry Test Answer all questions and box your final answers! You will receive 1 Communication mark for each answer boxed! Inquiry / Communication / 1. Potassium chlorate decomposes to form potassium chloride and oxygen gas. What mass of potassium chlorate is required to form 120.0 g of potassium chloride? /3 2. Consider the following balanced equation: 2 AgNO3 + CaCl2 Æ 2 AgCl + Ca(NO3)2. Calculate the amount of silver chloride, AgCl, that will be produced from 135.0 g of calcium chloride. /3 3. Consider the following balanced chemical equation: 3 Mg + 2 H3PO4 Æ 3 H2 + Mg3(PO4)2 Calculate the amount of magnesium phosphate that will be produced from 112.0 g of magnesium. /5 4. Iron(III) oxide can be formed by combining iron and oxygen gas. Determine the mass of iron(III) oxide produced when 48.2 g of oxygen gas reacts with 12.0 g iron. Fe(s) + O2(g) Æ Fe2O3(s) /7 5. Consider the following balanced equation: 2 Li3PO4 + 3 Zn(NO3)2 Æ Zn3(PO4)2 + 6 LiNO3 Determine the mass of lithium phosphate required to react completely with 415.0 g of zinc nitrate. /5 6. Consider the following balanced combustion reaction: CH4 + 2 O2 Æ CO2 + 2 H2O a) Determine the mass of water when 18.3 g of methane, CH4 and 4.5g oxygen react. /7 b) What is the mass of the leftover excess reagent?