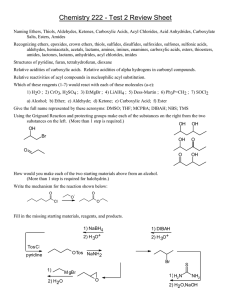
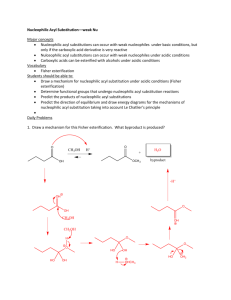
CARBOXYLIC ACID Esterification Fischer esterification is the preparation of an ester by treating a carboxylic acid with an alcohol in the presence of an acid catalyst such as sulfuric acid. Fischer esterification is reversible. An example of Fischer esterification is the treatment of acetic acid with ethanol in the presence of concentrated sulfuric acid, which gives ethyl acetate and water Reduction Lithium aluminum hydride (LiAlH4) reduces a carboxylic acid to a primary alcohol, although heating is required. Conversion to Acid Chlorides Acid chlorides are prepared from a carboxyl group by treatment with thionyl chloride (SOCl2). CARBOXYLIC DERIVATIVES Nucleophilic Acyl Addition This reaction can be carried out under basic conditions, in which a negatively charged nucleophile adds directly to the carbonyl carbon. The tetrahedral carbonyl addition intermediate formed then adds a proton from a proton donor, HA. Nucleophilic Acyl Substitution For functional derivatives of carboxylic acids, the fate of the tetrahedral carbonyl addition intermediate is quite different from that of aldehydes and ketones; the intermediate collapses to expel the leaving group (Lv) and regenerate the carbonyl group (break a bond to give stable molecules or ions). The major difference between nucleophilic acyl addition and nucleophilic acyl substitution is that aldehydes and ketones do not have a group that can leave as a relatively stable anion. Reaction with Water: Hydrolysis Esters are hydrolyzed only in the presence of acid or base. Acid is a catalyst. Base is required in an equimolar amount. In acid, the mechanism involves protonation of the acyl oxygen, attack by water to create the tetrahedral addition intermediate, transfer of a proton to the oxygen of the -OR group, and departure of the leaving alcohol.