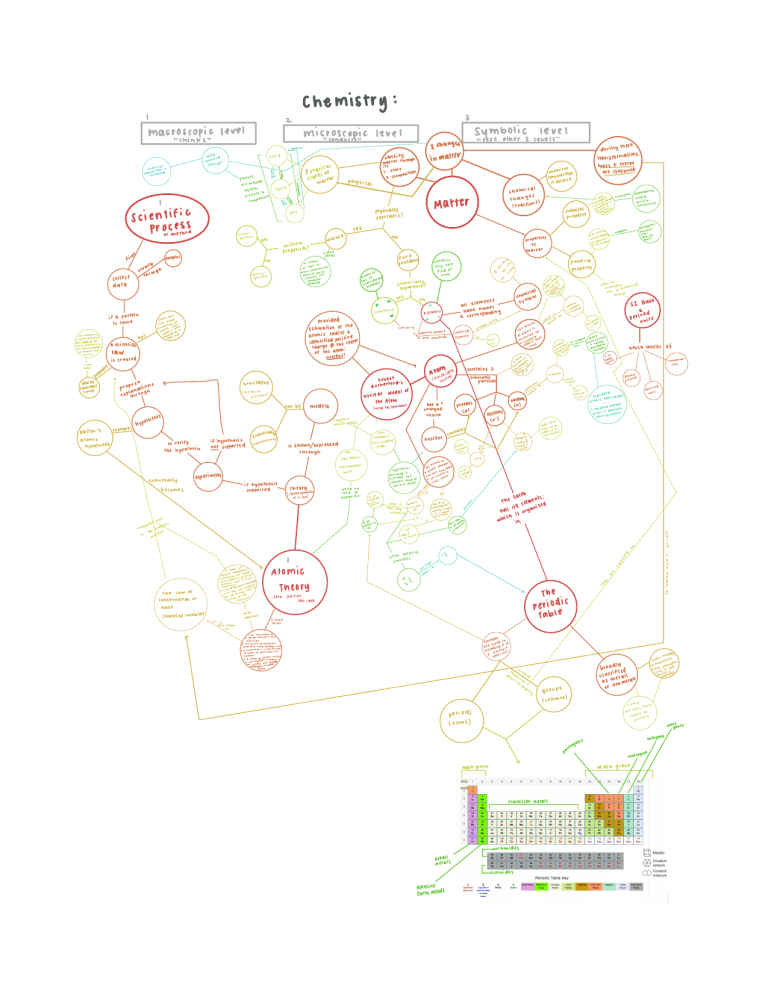
Scienti c Process Measurement; SI Base and Derived Units Hypothesis Theory Law Physical States (gas, liquid, solid) Elements Compounds Mixtures Physical Property (examples) Chemical Property (examples) Physical Change (examples) Chemical Change Law of Conservation of Mass Atomic Theory Law of Constant Composition Law of Multiple Proportions Atomic Models – Dalton (Billiard Ball), Thompson (Plum Pudding), Rutherford (Nuclear) Protons, Neutrons, Electrons Atomic Symbol Mass Number, Atomic Number Isotopes Ions Relate Atomic Mass AMU Periodic Table Group, Period Main Group Elements, Transition Elements, Inner Transition Elements Metals, Metalloids, Semi-Metals Alkali Metals, Alkaline Earth Metals, Chalcogens, Halogens, Noble Gases








