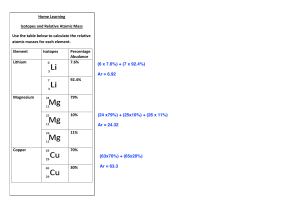
Isotopes and Relative Atomic Mass Isotopes Dalton’s Atomic Theory (pg 61) introduced a set of postulates, one of which said that all atoms of an element were identical. This was found to be incorrect due to the existence of isotopes. Isotopes are atoms of the same element, but with different mass numbers ie. different numbers of neutrons. So for example oxygen consists of three isotopes O 16, O 17, O 18. Each of these atoms has 8 protons ( and 8 electrons) but 8, 9 and 10 neutrons respectively. Relative Atomic Mass This is the weighted average of the mass of the isotopes and their abundance on a scale relative to 1/12 the mass of a carbon atom ( that is 1). It is a decimal value and is given on the periodic table. In the eg above, since O ends up with a relative atomic mass of 15.99, then the O 16 isotope must have a higher abundance ( % occurrence). Also an O atom is 1.33 x heavier than a C atom ( 12 compared to 16) . Calculations involve being given abundance ( %) of various isotopes , work out relative atomic mass, or being given relative atomic mass, work out abundance of the isotopes. Show working in all calculations please. Practice with these . 1. Thallium has two isotopes. The Th- 203 isotope is 29.5% abundant while the Th-205 is 70.5% abundant. Determine the relative atomic mass of thallium. ( would you expect the answer to be closer to 203 or closer to 205 ? ) 2. Tin ( Sn) has three isotopes : tin - 116 is 21% abundant, tin- 118 is 27% while tin- 120 is 52% abundant. What is the relative atomic mass of tin? ( Sn) 3. The element neon has three isotopes. Ne- 20 is 90.9% abundant, the Ne-21 is 0.3% abundant, while Ne- 22 is 8.8% abundant. What is the atomic mass of neon? WORKING OUT ABUNDANCE ( %) 4. Copper has two isotopes Cu-63 and Cu -65. If the relative atomic mass of Cu is 63.5 ( amu atomic mass units), what is the occurrence ( % ) of each isotope? ( would you expect Cu 63 or Cu 65 to have the higher %? ) 5. Rubidium has two isotopes Rb- 85 and Rb-87. If the relative atomic mass of Rb is 85.6, what is the abundance of each isotope?