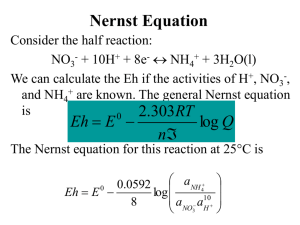
19 Applications of Standard Electrode Potentials (1) Calculating thermodynamic cell potentials (2) Calculating equilibrium constants for redox reactions (3) Constructing redox titration curves 19A Calculating Potentials of Electrochemical Cells Ecell = Eright – Eleft Ex. 19-1 Calculate the thermodynamic potential of the following cell and the free energy change associated with the cell reaction. Cu|Cu2+(0.0200 M)∥Ag+(0.0200 M)|Ag Ag+ + e- → Ag(s) Cu2+ + 2e- → Cu(s) E Ag + /Ag = 0.799 − 0.0592 log E Cu 2 + /Cu = 0.337 − Eº = 0.799 V Eº = 0.337 V 1 = 0.6984V 0.0200 0.0592 1 log = 0.2867V 0.0200 2 Ecell = EAg+ - ECu2+ = 0.6984 - 0.2867 = + 0.412 V ΔG for Cu(s) + 2Ag+ → Cu2+ + 2Ag(s) ΔG = -nFEcell = -2 × 96485 C × 0.412 V = -79500J (18.99 Kcal) Ex. 19-2 Calculate the thermodynamic potential of the cell Ag|Ag+(0.0200 M)∥Cu2+(0.0200 M)|Cu Ecell = ECu2+ - EAg+ = 0.2867 - 0.6984 = – 0.412 V Ex. 19-3 Calculate the potential of the following cell and indicate whether it is galvanic or electrolytic (see Fig. 19-1). Pt|UO22+(0.0150 M),U4+(0.200 M), H+(0.0300 M)∥ Fe2+(0.0100 M), Fe3+(0.0250 M),|Pt Eº = 0.771 V Fe3+ + e- → Fe2+ 2+ + 4+ UO2 + 2H2O Eº = 0.334 V + 4H + 2e → U E cathode E° anode [Fe 2+ ] 0.0100 0 . 771 0 . 0592 log = 0.771 − 0.0592 log = − 0.0250 [Fe 3+ ] = 0.771-(-0.0236) = 0.7946 V 0.200 [ U 4+ ] 0.0592 0.0592 = 0.334 − = 0.334 − log log 2+ + 4 2 2 0.0150 × 0.0300 4 [ UO 2 ][H ] = 0.334 - (0.2136) = 0.1204 V 127 Ecell = Ecathode – Eanode = 0.7946 – 0.1204 = +0.674 V ( positive sign → galvanic) Fig. 19-2 Cell without liquid junction for ex. 19-4 Fig. 19-1 Cell for ex. 19-3 Ex. 19-4 Calculate the theoretic potential for Ag|AgCl(sat'd), HCl(0.0200 M) | H2(0.800 atm), Pt 2H+ + AgCl(s) 2e+ H2(g) → e- → E°cathode = 0.000 − Eº = 0.000 V +Cl- Ag(s) Eº = 0.222 V 0.0592 0.800 = −0.0977V log 2 2 0.0200 Eanode = 0.222 – 0.0592 log 0.0200 = 0.3226 V Ecell = – 0.0977 – 0.3226 = – 0.420 V The negative sign means that the cell reaction 2H+ + Ag(s) Cl- + → H2(g) + AgCl(s) is nonspontaneous, and thus the cell is electrolytic. Ex. 19-5 Calculate the potential for the following cell using (a) concentration and (b) activities: Zn|ZnSO4(x M), PbSO4(sat’d)|Pb, where x = 5.00 × 10-4, 2.00 × 10-3, 1.00 × 10-2 and 5.00 × 10-2, (a) In a neutral solution, [SO42-] = cZnSO4 = x = 5.00 × 10-4, + PbSO4 Zn2+ + 2e2e- ⇔ ⇔ Pb(s) + SO42Zn(s) Eº = -0.350 V Eº = -0.763 V 128 E PbSO3/Pb = −0.350 − E Zn 2+ /Zn = −0.763 − 0.0592 log(5.00 × 10 −4 ) = −0.252V 2 0.0592 1 log = −0.860V 2 5.00 × 10 − 4 Ecell = Eright – Eleft = -0252 – (-0.860) = +0.608 V (b) Calculate activity coefficient for Zn2+ and SO421 2 μ = (5.00 × 10 −4 × 2 2 + 5.00 × 10 −4 × 2 2 ) = 2.00 × 10 −3 − log γ SO 2 − = 4 0.51× 2 2 2.00 × 10 −3 1 + 3.3 × 0.4 2.00 × 10 −3 E PbSO 4 /Pb = −0.350 − E Zn 2 + /Zn = −0.763 − = 8.61 × 10 − 2 , γ SO 2- = 0.820, γ Zn 2 + = 0.825 4 0.0592 log(0.820 × 5.00 × 10 −4 ) = −0.250V 2 0.0592 1 log = −0.863V 0.825 × 5.00 ×10 −4 2 Ecell = Eright – Eleft = -0250 – (-0.863) = + 0.613 V Table 19-1 Effect of Ionic Strength on the Potential of a Galvanic Cell E, V, based on E, V, Experimental E, V, based on μ, Ionic [ZnSO4], M Concentration Activity Values Strength -4 5.00 × 10 2.00 × 10-3 1.00 × 10-2 2.00 × 10-2 5.00 × 10-2 2.00 × 10-3 8.00 × 10-3 4.00 × 10-2 8.00 × 10-2 2.00 × 10-1 0.608 0.573 0.531 0.513 0.490 0.613 0.582 0.550 0.537 0.521 0.611 0.583 0.553 0.542 0.529 Ex. 19-6 Calculate the potential required to initiate deposition of Cu from a solution that is 0.010 M in CuSO4 and contains sufficient H2SO4 to give a pH of 4.00. Eº = +1.229 V O2(g) + 4H+ + 4e- ⇔ 2H2O 2+ + 2e ⇔ Cu(s) Eº = +0.337 V Cu 0.0592 1 E Cu 2 + /Cu = +0.337 − log = +0.278V 2 0.010 E O 2 /H 2 O = +1.229 − 1 0.0592 1 0.0592 = − = +0.992V log 1 . 229 log pO2 × [H + ]4 1 atm × 10- 4 4 4 Ecell = Eright – Eleft = +0.278 – 0.992 = -0.714 V ⇒ The cell reaction Cu2+ + 2H2O ⇔ O2(g) + 4H+ + Cu(s) is nonspontaneous and that to cause Cu to be deposited, we must apply a cathode potential more negative than -0.714 V 129 19B Determining Standard Potentials Experimentally Ex. 19-7 Pt,H2(1.00 atm)|HCl(3.215×10-3 M), AgCl(sat’d)|Ag Eº = +0.52053 V Calculate the Eº for the half-reaction AgCl(s) + e ⇔ Ag(s) +Cl0 E right = E AgCl − 0.0592 log(γ Cl- )(c HCl ) 1 Eleft = EH0 + /H − 0.0592 log H+ + e- ⇔ ½ H2(g) 2 p H22 (γ H + )(cHCl ) Ecell = Eright – Eleft 1 ⎡ ⎤ 2 p H 0 0 2 ⎢ ⎥ = [ E AgCl − 0.0592 log(γ Cl− )(γ HCl )] − EH + /H − 0.0592 log 2 ⎢ (γ H + )(cHCl ) ⎥ ⎣ ⎦ (γ + )(cHCl ) 0 = E AgCl − 0.0592 log(γ Cl− )(γ HCl ) − 0.000 − 0.0592 log H 1 p H22 Ecell = 0.52053 = E E 0 AgCl 0 AgCl − 0.0592 log = 0.52053 + 0.0592 log (γ H + )(γ Cl− )(cHCl ) 2 1 p H22 (0.945)(0.939)(3.215 × 10 −3 ) 2 1.00 1 = 0.2223 ≈ 0.222 V 2 19C Calculating Redox Equilibrium Constants Cu(s) + 2Ag+ Cu2+ + → 2Ag(s) K eq = [Cu 2 + ] [Ag + ]2 Cu|Cu2+(x M)∥Ag+(y M)|Ag at chemical equilibrium Ecell = 0 = Eright – Eleft = EAg – ECu or Eright = Eleft = EAg = ECu Eox1 = Eox2= Eox3 = Eox4 E 0Ag − 0.0592 1 0.0592 1 log log = E 0Cu − + 2 2 2 [Ag ] [Cu 2+ ] 2Ag+ + E 0 Ag −E 0 Cu 2e- → 2Ag(s) Eº = 0.799V 0.0592 1 0.0592 1 0.0592 [Cu 2+ ] = log − log = log 2 [ Ag + ]2 2 [Cu 2+ ] 2 [Ag + ]2 0.0592 log K eq = 2 130 2 (E oAg − E oCu ) [Cu 2 + ] = log = log K eq [Ag + ] 2 0.0592 ln K eq 0 ΔG 0 nFE cell =− = RT RT → n (E oB − E oA ) log K eq = 0.0592 at 25°C log K eq 0 n (E oright − E oleft ) nE cell = = 0.0592 0.0592 Ex. 19-8 Calculate the Keq for the reaction Cu(s) + 2Ag+ → Cu2+ + 2Ag(s) log K eq = log [Cu 2 + ] [Ag + ]2 2 (0.799 − 0.337) = 15.61 0.0592 = Keq = antilog 15.61 = 4.1 × 1015 Ex. 19-9 Calculate the Keq for the reaction 2Fe3+ + 3I- → 2Fe2+ + I32Fe3+ + I3- E + Fe 3+ /Fe 2+ 2e2e- → → = E° 2Fe2+ 0.771 V 3I- Fe 3+ /Fe 2+ 0.536 V 0.0592 [Fe 2 + ]2 log − 2 [ Fe3+ ]2 0.0592 [ I - ]3 E - - = E° - - − log I 3 /I I 3 /I 2 [ I3- ] E at equilibrium E° Fe3+ /Fe 2 + Fe3+ /Fe 2 + =E I3- /I - 0.0592 [ Fe 2 + ]2 = 0.0592 [I - ]3 − log E° - - − log I 3 /I 2 2 [Fe3+ ]2 [I3- ] 2(E ° Fe3+ /Fe 2 + − E °- ) I3 0.0592 = log [Fe 2 + ]2 [Fe3+ ]2 − log [I - ]3 [I3- ] = log [Fe 2 + ]2 [I3- ] [Fe3+ ]2 [I - ]3 2(E ° 3+ 2 + − E °- - ) 2+ 2 [Fe ] [I3 ] Fe /Fe I3 / I = log 3+ 2 - 3 0.0592 [Fe log K eq = 2(E° Fe ] [I ] 3+ /Fe 2+ − E °- I3 / I- ) = 0.0592 Keq = antilog 7.94 = 8.7 × 107 2(0.771 − 0.536) = 7.94 0.0592 131 Ex. 19-10 Calculate the Keq for the reaction 2MnO4- + 3Mn2+ + 2H2O → 5MnO2(s) + 4H+ 2MnO4- + 8H+ + 6e- →2MnO2(s) + 4H2O Eº = 1.695 V 3MnO2(s) + 12H+ + 6e- → 3Mn2+ + 6H2O Eº = 1.23 V EMnO4-/MnO2 = EMnO2/Mn2+ 1.695 - 0.0592 1 0.0592 [Mn 2 + ]3 log log = 1.23 − log 6 6 [MnO-4 ]2 [H + ]8 [H + ]12 6(1.695 - 1.23) [H + ]12 [H + ]12 1 = log + log = log 0.0592 [MnO -4 ] 2 [H + ] 8 [Mn 2 + ] 3 [MnO -4 ] 2 [Mn 2 + ] 3 [H + ] 8 log K eq = log [H + ]4 [MnO -4 ]2 [Mn 2 + ]3 = 47.1 Keq = antilog 47.1 = 1 × 1047 19D Constructing Redox Titration Curves 19D-1 Electrode Potentials during Redox Titrations Fe2+ + Ce4+ → Fe3+ + Ce3+ ECe4+/Ce3+ = EFe3+/Fe2+ = Esystem = EIn SHE∥Ce4+, Ce3+, Fe3+, Fe2+ | Pt Most end point in oxidation/reduction titrations are based on the rapid changes in Esystem that occur at or near chemical equivalence. Before the equivalence point, Esystem calculations are easiest to make using the Nernst equation for the analyte. Beyond the equivalence point, the Nernst equation for the titrant is more convenient. Equivalence-point Potentials at the equivalence point [Fe 2 + ] [Ce3+ ] o o and E eq = E 3+ − 0.0592 log E eq = E 4 + − 0.0592 log 4+ 3+ Ce Fe [Ce [Fe ] [Ce3+ ][Fe 2 + ] o o = E o 4 + + E o 3+ 2E eq = E 4 + + E 3+ − 0.0592 log Ce Fe Ce Fe [Ce 4 + ][Fe3+ ] 3+ 3+ 2+ 4+ ([Fe ] = [Ce ], [Fe ] = [Ce ]) E eq = 132 Eo Ce 4 + + Eo Fe3+ 2 ] Ex. 19-11 Obtain an expression for the equivalence-point potential in the titration of 0.0500 M U4+ with 0.1000 M Ce4+. Assume both solutions are 1.0 M in H2SO4. U4+ + 2Ce4+ UO22+ + Ce4+ 4H+ e- + + 2H2O → 2e- + → → Ce3+ UO22+ U4+ + 2Ce3+ + 2H2O + 4H+ Eº = 0.334 V (formal potential) Eº' = 1.44 V 0.0592 E eq = E ° 2 + − log UO 2 2 [U 4+ ] [ UO 22 + ][H + ]4 [Ce3+ ] 0.0592 °' log E eq = E 4 + − 4+ Ce 1 [Ce (a) (b) ] [ U 4 + ][Ce3+ ] 2a + b → 3E eq = 2E ° 2 + + E °' 4 + − 0.0592 log UO 2 Ce [ UO 2 + ][Ce 4 + ][H + ]4 2 [U4+] = [Ce4+]/2 at equivalence E eq = = 2E ° UO 22 + + E °' Ce 4 + 3 2E ° UO 22 + + E °' Ce 4 + 3 and [UO22+] = [Ce3+]/2 − 2[Ce 4 + ][Ce3+ ] 0.0592 log 3 2[Ce3+ ][Ce 4 + ][H + ]4 − 0.0592 1 log 3 [H + ]4 pH dependent 19D-2 The Titration Curves Titration of 50.00 mL of 0.0500 M Fe2+ with 0.1000 M Ce4+ in a medium that is 1.0 M in H2SO4 at all times. Ce4+ + Fe2+ → Ce4+ + e- → Ce3+ Fe3+ + e- → Fe2+ Initial Potential Fe3+ + Ce3+ Eº' = 1.44 V (1M H2SO4) Eº' = 0.68 V (1M H2SO4) Potential After the Addition of 5.00 mL of Cerium(IV) [Fe3+ ] = 5.00 × 0.100 0.500 − [Ce 4 + ] = − [Ce 4 + ] 50.00 + 5.00 55.00 [ Fe 2 + ] = 50.00 × 0.0500 − 5.00 × 0.100 2.00 + [Ce 4 + ] = + [Ce 4 + ] 55.00 55.00 [Fe3+] ≒ 0.500/55.00 E system = +0.68 − and [Fe2+] ≒ 2.00/55.00 0.0592 2.00 / 55.00 log = 0.64 V 1 0.500 / 55.00 133 Equilvalence-Point Potential E 0' E eq = Ce 4+ + E 0' 3 + Fe 2 = 1.44 + 0.68 = 1.06 V 2 Potential After the Addition of 25.10 mL of Cerium(IV) [Ce3+ ] = 25.00 × 0.100 2.500 − [Fe2 + ] ≈ 75.10 75.10 [Ce 4 + ] = 25.10 × 0.1000 − 50.00 × 0.0500 0.010 + [Fe 2 + ] ≈ 75.10 75.10 E = +1.44 − 0.0592 [Ce 3+ ] 0.0592 2.500 / 75.10 log = 1.44 − log = 1.30 V 1 1 0.010 / 75.10 [Ce 4 + ] Table 19-2 Electrde Potential versus SHE in Titrations with 0.100 M Ce4+ Reagent Volume, mL 5.00 15.00 20.00 24.00 24.90 Potential, vs, SHE 50.00 mL of 0.0500 M Fe2+ 50.00 mL of 0.02500 M U4+ 0.64 0.316 0.69 0.339 0.72 0.352 0.76 0.375 0.82 0.405 25.00 1.06 25.10 26.00 30.00 1.30 1.36 1.40 Equivalence Point 0.703 1.30 1.36 1.40 Oxidation/reduction curves are independent of the concentration of the reactants except when the solution is very dilute. Fig. 19-3 Titration curves for 0.1000M Ce4+ titration. A: Titration of 50.00 mL of 0.05000 M Fe2+. B: Titration of 50.00 mL of 0.02500 M U4+. 134 19D-3 Effect of Variables on Redox Titration Curves Reactant concentration titration curves are usually independent of analyte and reagent conc. Completeness of the Reaction completeness of the reaction↑ → change in Esystem in the equivalence-point region ↑ Fig. 19-5 Effect of titrant electrode potential on reaction completeness. The standard electrode potential for the analyte (EA0) is 0.200V; starting with curve A, standard electrode potentials for the titrant (ET0) are 1.20, 1.00, 0.80, 0.60 and 0.40, respectively. Both analyte and titrant undergo a one-electron change. 19E Oxidation/Reduction Indicators *Specific Indicators: react with one of the participants in the titration to produce a color. starch: form a deep blue complex with iodine KSCN: form a red Fe(III)/thiocyanate complex with Fe(III) *General Redox Indicators: respond to the potential of the system. Inox + ne- a color change → 0 E = EIn − ox /In red Inred Inox → [In ] 0.0592 log red n [In ox ] Inred 0.0592 [In red ] 1 [In red ] 0 E = EIn ± ≤ → ≥ 10 n [In ox ] 10 [In ox ] For a typical indicator to undergo a useful transition in color, the titrant must cause a change of 0.118/n V in the potential of the system. For many indicators, n is 2, so a change of 0.059 V is sufficient. Iron(II) Complexes of Orthophenanthrolines (phen)3Fe3+ + pale blue e- ⇔ (phen)3Fe2+ red 135 Table 19-3 Selected Oxidation/Reduction Indicators Color Transition Indicator Potential, V Oxidized Reduced 5-Nitro-1,10Pale blue Red-violet +1.25 phenanthroline iron(II) complex 2,3’-Diphenylamine Blue-violet Colorless + 1.12 dicarboxylic acid 1,10-Phenanthroline Pale blue Red + 1.11 iron(II) complex 5-Methyl-1,10Pale blue Red + 1.02 phenanthroline iron(II) complex Erioglaucin A Blue-red Yellow-green + 0.98 Diphenylamine Red-violet Colorless + 0.85 sulfonic acid Diphenylamine Violet Colorless + 0.76 p-Ethoxychrysoidine Yellow Red + 0.76 Methylene blue Blue Colorless + 0.53 Indigo tetrasulfonate Blue Colorless + 0.36 Phenosafranine Red Colorless + 0.28 N Conditions 1M H2SO4 7-10 M H2SO4 1M H2SO4 1M H2SO4 0.5M H2SO4 Dilute acid Dilute acid Dilute acid 1M acid 1M acid 1M acid N 2+ Fe N N 3 1,10-phenanthroline NO2 ferroin (phen)3Fe2+ N H3C N N N 5-nitro-1,10- phenanthroline 5-methyl-1,10- phenanthroline 136