Activity and Activity Coefficient calculations
advertisement

ACTIVITY
Activity Coefficients
• No direct way to measure the effect of a
single ion in solution (charge balance)
• Mean Ion Activity Coefficients – determined
for a salt (KCl, MgSO4, etc.)
g±KCl = [(gK)(gCl)]1/2
Ksp= g±KCl2(mK+)(mCl-)
• MacInnes Convention gK = gCl= g±KCl
– Measure other salts in KCl electrolyte and
substitute g±KCl in for one ion to measure the
other ion w.r.t. g±KCl and g±salt
Mean Ion Activity Coefficients versus Ionic Strength
Debye-Hückel
log g i Az
2
i
I
Azi2 I
log g i
1 Bai I
• Assumes ions interact coulombically, ion
size does not vary with ionic strength, and
ions of same sign do not interact
• A, B often presented as a constant, but:
A=1.824928x106r01/2(T)-3/2, B=50.3 (T)-1/2
Where is the dielectric constant of water
and r is the density
Higher Ionic Strengths
• Activity coefficients decrease to minimal
values around 1 - 10 m, then increase
– the fraction of water molecules surrounding
ions in hydration spheres becomes significant
– Activity and dielectric constant of water
decreases in a 5 M NaCl solution, ~1/2 of
the H2O is complexed, decreasing the activity
to 0.8
– Ion pairing increases, increasing the activity
effects
Extended Debye-Hückel
2
I
2 Azi
log g i Az
0.3I
1 Ba i I
• Adds a correction term to account for
increase of gi after certain ionic strength
• Truesdell-Jones (proposed by Huckel in
1925) is similar:
Azi2 I
log g i
bI
1 Ba i I
Davies Equation
I
log g i Az
0.3I
1 I
2
• Lacks ion size parameter –only really
accurate for monovalent ions
• Often used for Ocean waters, working
range up to 0.7 M (avg ocean water I)
Specific Ion Interaction theory
log( g i ) z 2 D (i, j, I )m( j )
k
• Ion and electrolyte-specific approach for
activity coefficients
• Where z is charge, i, m(j) is the molality of
major electrolyte ion j (of opposite charge to
i). Interaction parameters, (i,j,I) describes
interaction of ion and electrolyte ion
• Limited data for these interactions and
assumes there is no interaction with neutral
species
Pitzer Model
ln g i zi2 fy Dij ( I )m j Eijk m j mk ...
i
ijk
• At ionic strengths above 2-3.5, get +/+, -/and ternary complexes
• Terms above describe binary term, fy
describes interaction between same or
opposite sign, terms to do this are called
binary virial coefficients
• Ternary terms and virial coefficients refine
this for the activity coefficient
Setchenow Equation
•
•
log gi=KiI
For molecular species (uncharged) such as
dissolved gases, weak acids, and organic
species
Ki is determined for a number of important
molecules, generally they are low, below
0.2 activity coefficients are higher,
meaning mi values must decline if a
reaction is at equilibrium “salting out”
effect
Half Reactions
• Often split redox reactions in two:
– oxidation half rxn e- leaves left, goes right
• Fe2+ Fe3+ + e-
– Reduction half rxn e- leaves left, goes right
• O2 + 4 e- 2 H2O
• SUM of the half reactions yields the total
redox reaction
4 Fe2+ 4 Fe3+ + 4 eO2 + 4 e- 2 H2O
4 Fe2+ + O2 4 Fe3+ + 2 H2O
ELECTRON ACTIVITY
• Although no free electrons exist in solution, it is useful
to define a quantity called the electron activity:
pe log ae
• The pe indicates the tendency of a solution to donate or
accept a proton.
• If pe is low, there is a strong tendency for the solution to
donate protons - the solution is reducing.
• If pe is high, there is a strong tendency for the solution to
accept protons - the solution is oxidizing.
THE pe OF A HALF REACTION - I
Consider the half reaction
MnO2(s) + 4H+ + 2e- Mn2+ + 2H2O(l)
The equilibrium constant is
K
aMn2
4
H
a a
2
e
Solving for the electron activity
aMn2
ae 4
Ka
H
1
2
DEFINITION OF Eh
Eh - the potential of a solution relative to the SHE.
Both pe and Eh measure essentially the same thing.
They may be converted via the relationship:
pe
Eh
2.303RT
Where = 96.42 kJ volt-1 eq-1 (Faraday’s constant).
At 25°C, this becomes
pe 16.9 Eh
or
Eh 0.059 pe
Free Energy and Electropotential
• Talked about electropotential (aka emf, Eh)
driving force for e- transfer
• How does this relate to driving force for any
reaction defined by DGr ??
DGr = - nE
– Where n is the # of e-’s in the rxn, is Faraday’s
constant (23.06 cal V-1), and E is electropotential (V)
• pe for an electron transfer between a redox
couple analagous to pK between conjugate acidbase pair
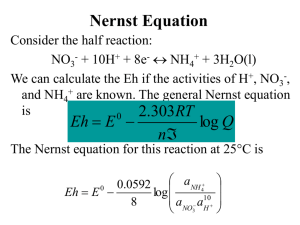
Nernst Equation
Consider the half reaction:
NO3- + 10H+ + 8e- NH4+ + 3H2O(l)
We can calculate the Eh if the activities of H+, NO3-,
and NH4+ are known. The general Nernst equation
is
2.303RT
0
Eh E
log Q
n
The Nernst equation for this reaction at 25°C is
aNH
0
.
0592
4
Eh E 0
log
aNO a10
8
3 H
Let’s assume that the concentrations of NO3- and
NH4+ have been measured to be 10-5 M and
310-7 M, respectively, and pH = 5. What are the
Eh and pe of this water?
First, we must make use of the relationship
DG
E
n
0
o
r
For the reaction of interest
DrG° = 3(-237.1) + (-79.4) - (-110.8)
= -679.9 kJ mol-1
679.9
0
E
0.88 volts
(8)( 96.42)
C2HO
UPPER STABILITY LIMIT OF
WATER (Eh-pH)
To determine the upper limit on an Eh-pH
diagram, we start with the same reaction
1/2O2(g) + 2e- + 2H+ H2O
but now we employ the Nernst eq.
0.0592
1
0
Eh E
log
n
pO aH2
1
2
2
0.0592
1
Eh E
log 12 2
2
pO2 aH
0
DG
( 237.1)
E
1.23 volts
n
(2)( 96.42)
0
0
r
Eh 1.23 0.0296 log pO22 aH2
1
Eh 1.23 0.0148 log pO2 0.0592 pH
As for the pe-pH diagram, we assume that
pO2 = 1 atm. This results in
Eh 1.23 0.0592 pH
This yields a line with slope of -0.0592.
LOWER STABILITY LIMIT OF
WATER (Eh-pH)
Starting with
H+ + e- 1/2H2(g)
we write the Nernst equation
1
2
p
0
.
0592
H2
0
Eh E
log
1
aH
We set pH2 = 1 atm. Also, DGr° = 0, so E0 =
0. Thus, we have
Eh 0.0592 pH
Construction of these diagrams
• For selected reactions:
Fe2+ + 2 H2O FeOOH + e- + 3 H+
3
a
0.0592 H
0
Eh E
log
a 2
1
Fe
How would we describe this reaction on a 2-D
diagram? What would we need to define or
assume?
• How about:
• Fe3+ + 2 H2O FeOOH(ferrihydrite) + 3 H+
Ksp=[H+]3/[Fe3+]
log K=3 pH – log[Fe3+]
How would one put this on an Eh-pH diagram,
could it go into any other type of diagram
(what other factors affect this equilibrium
description???)
INCONGRUENT
DISSOLUTION
• Aluminosilicate minerals usually dissolve
incongruently, e.g.,
2KAlSi3O8 + 2H+ + 9H2O
Al2Si2O5(OH)4 + 2K+ + 4H4SiO40
• As a result of these factors, relations among
solutions and aluminosilicate minerals are often
depicted graphically on a type of mineral
stability diagram called an activity diagram.
ACTIVITY DIAGRAMS: THE
K2O-Al2O3-SiO2-H2O SYSTEM
We will now calculate an activity diagram for the
following phases: gibbsite {Al(OH)3}, kaolinite
{Al2Si2O5(OH)4}, pyrophyllite
{Al2Si4O10(OH)2}, muscovite
{KAl3Si3O10(OH)2}, and K-feldspar {KAlSi3O8}.
The axes will be a K+/a H+ vs. a H4SiO40.
The diagram is divided up into fields where only
one of the above phases is stable, separated by
straight line boundaries.
6
Quartz
7
Amorphous
silica
Activity diagram showing the stability relationships among some
minerals in the system K2O-Al2O3-SiO2-H2O at 25°C. The dashed
lines represent saturation with respect to quartz and amorphous silica.
Muscovite
log (aK+/aH+)
5
K-feldspar
4
3
Gibbsite
Kaolinite
2
1
Pyrophyllite
0
-6
-5
-4
-3
log aH SiO 0
4
4
-2
-1
Seeing this, what are the reactions these lines represent?
2
0
Gibbsite
Al
+++
, T = 25 C , P = 1. 013 bars , a [ H 2O] = 1
-4
-
-6
Al(OH)4
-8
++
+++
+
AlOH
Al(OH)2
Diagram A l
log a Al
+++
-2
25oC
-10
2
3
4
5
6
7
pH
8
9
10
11
12
Greg Mon Nov 01 2004