Uploaded by
Christopher Beda
Acid-Base Reactions Worksheet: Chemistry Indicators & pH Scale
advertisement

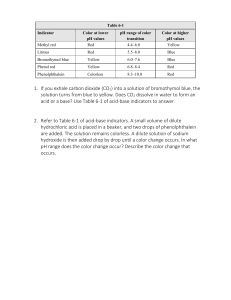
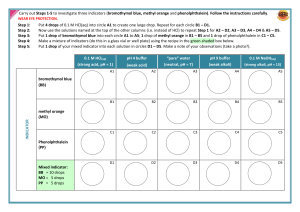
KISS Resources for NSW Syllabuses & Australian Curriculum KEEP IT SIMPLE SCIENCE ® keep it simple science Worksheet 1 Part 1 Chemistry Module 6 Acid-Base Reactions WORKSHEETS Acids, Bases & Indicators Fill in the blank spaces Student Name.......................................... Acids and bases are chemical a)................................ If you add one to the other, they b).............................. each other. The colour changes for the following indicators need to be learnt. Indicator Acid Neutral Base Acidity is measured by the c)............... scale. On this scale, a neutral substance has a value of d)....... Values above this are e).................................. substances, while values below indicate f). ........................................ substances. Litmus Indicators are chemicals which g).............................. j)............. Phenolphthalein l)........... purple k).............. ................ ................ Methyl orange m)............. ................ ............. What is a base …………………………………………………………………… ……………………………………………………………… ........................ according to the h). ........................ of What is an acid _______________________________________________ _______________________________________________ _______________________________________________ ____________________________________________. the solution they are in. Part 2 1. Each solution listed below has been tested with one or more indicators, and the colour is given. For each, state if it is acidic, neutral or basic. Solution A: Phenolphthalein is clear. Methyl orange is red. Practice Questions 2. A household substance most likely to be stronglybasic/alkaline is A. vinegar. B. soap. C. sugar. D. milk. a) What is meant by an “acid-base indicator”? Solution B: Phenolphthalein is pink. Methyl orange is yellow. Strong acids have a pH of………………………….. Solution C: Phenolphthalein is clear. Methyl orange is yellow. Solution D: Methyl orange is yellow. Solution E: Phenolphthalein is clear. Litmus is red/pink. Weak acids have a pH of …………………………… Reaction of an acid and a base is called ………………………………………………………………… ………………………………………………………………… ………………………………………………………………… …….. Reaction of a base and acid gives you …………………..and …………………………………….. …