Acids and Bases Name ______________________________ Applied Chemistry
advertisement
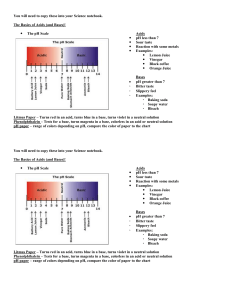
Acids and Bases Applied Chemistry Name ______________________________ Date ______________ Period _____ I. Acids A. Definition: A substance that produces ____________________________ in water. General reaction in water: HA → H+ + AB. Formula always has ________________ in its formula and it is usually written ____________. 1. Examples: C. Strength: a measure of the amount of _____________ formed in water. 1. Strong: __________________ Example: _________________ 2. Weak: ___________________ Example: _________________ D: Concentration: relates to the amount of ________________ present. 1. Concentrated: _________________________________________________ 2. Dilute: _______________________________________________________ E. Properties 1. _________________ taste 2. Changes the color of an _____________________ a. Indicator: a substance that indicates whether a substance is an _____________ or ______________ by ________________. b. Examples: Litmus is __________ in acid. Phenolphthalein is ________________ in acid. 3. Conducts _____________________. 4. Reacts with metals to form ____________________ gas. Example: HCl + Zn → H2 + ZnCl2 II. Bases A. Definition: a substance that produces ______________________ in water. General reaction: XOH → X+ + OHB. Formula: always has a ____________________ and _______________ in its formula. Examples: C. Properties 1. _______________ taste 2. Change the color of an ______________________. Example: Litmus is ________________ in base. Phenolphthalein is ___________ in base. 3. Conducts _____________________. 4. Feels _____________________. III.. pH of Solutions 1. pH is a measure of _________________________ concentration. 2. The pH scale ranges from _______ to ________. -pH below 7 is ____________________ -pH of 7 is _______________________ -pH above 7 is ______________________