Isotopes
advertisement

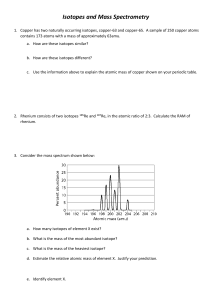
Name:____________________ Date:____________ Period:___________ ISOTOPES WORKSHEET There are 2 ways to represent isotopes: 1. Elements name-mass number Example lithium-7 2. Mass Number 7 Symbol Atomic Number Li 3 I. Using a periodic table, represent each of the following isotopes in the two ways shown above 1. 50 p+ 72 n 50 e- 2. 21 e21 p+ 25 n 3. 9 p+ 9 e12 n a)______________ a)______________ a)______________ b)______________ b)______________ b)______________ 4. 14 n 15 e15 p+ 5. a)______________ a)______________ a)______________ b)______________ b)______________ b)______________ 82 e127 n 82 p+ 6. 140 n 88 p+ 88 e- II. The Isotopes of Hydrogen 7. How many isotopes does hydrogen have?_________ 8. How are they all alike?_________________________________ 9. List all three and give both types of representations. a. ________________________________________________________________ b. ________________________________________________________________ c. ________________________________________________________________ Give the name of each isotope in these formats: Carbon-12 and 12 C 6 1. 8 p+, 8 n0, 8 e- ______________________ and __________ 2. 14 p+, 14 n0, 14 e- ______________________ and __________ 3. 22 p+, 25 n0, 22 e- ______________________ and __________ 4. 78 p+, 117 n0, 78 e- ______________________ and __________ 5. 87 p+, 136 n0, 87 e- ______________________ and __________ 6. 40 p+, 51 n0, 40 e- ______________________ __________ and What is the mass number in each of the following: 7. 60 Co _____ 24 8. 27 Na _____ 206 9. 11 Pb _____ 10. 82 40 K _____ 19 Determine the number of neutrons in the following: 11. carbon-14 _____ 14. 60 Co _____ 27 12. barium-139 _____ 15. 24 Na _____ 11 16. 206 13. lead-204 _____ Pb _____ 82 17. 40 K _____ 19 List the number of protons, neutrons, electrons, the atomic number Z, and the mass number for the most common isotopes of the following elements: 18. Li 19. Mg 20. Hg 21. C 22. I P __________ __________ __________ __________ _________ E __________ __________ __________ __________ _________ N __________ __________ __________ __________ _________ Z __________ __________ __________ __________ _________ A __________ __________ __________ __________ _________