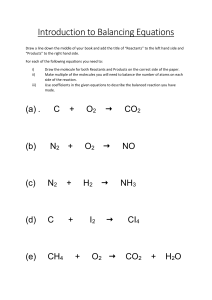
10 Science Quarter 4 – Module 3: Chemical Reactions Science – Grade 10 Alternative Delivery Mode Quarter 4 – Module 3: Chemical Reactions First Edition, 2020 Republic Act 8293, section 176 states that: No copyright shall subsist in any work of the Government of the Philippines. However, prior approval of the government agency or office wherein the work is created shall be necessary for the exploitation of such work for a profit. Such agency or office may, among other things, impose as a condition the payment of royalties. Borrowed materials (i.e., songs, stories, poems, pictures, photos, brand names, trademarks, etc.) included in this module are owned by their respective copyright holders. Every effort has been exerted to locate and seek permission to use these materials from their respective copyright owners. The publisher and authors do not represent nor claim ownership over them. Published by the Department of Education Secretary: Leonor Magtolis Briones Undersecretary: Diosdado M. San Antonio Development Team of the Module Writer: Catherine C. Manzano Editor: Ann Christian A. Francisco Reviewer: William M. Cinense Illustrator: Wensdy S. Casio Layout Artist: Aljhon U. Febrero Management Team: May B. Eclar, CESO III Librada M. Rubio, PhD Ma. Editha R. Caparas, EdD Nestor R. Nuesca, EdD Larry B. Espiritu, PhD Rodolfo A. Dizon, PhD Mary Queen P. Orpilla, PhD Printed in the Philippines by ________________________ Department of Education – Region III Office Address: Telefax: E-mail Address: Matalino St., Diosdado Macapagal Center Maimpis, City of san Fernando (P) (045) 598-8580 to 89 region3@deped.gov.ph 10 Science Quarter 4 – Module 3: Chemical Reactions Introductory Message This Self-Learning Module (SLM) is prepared so that you, our dear learners, can continue your studies and learn while at home. Activities, questions, directions, exercises, and discussions are carefully stated for you to understand each lesson. Each SLM is composed of different parts. Each part shall guide you step-by-step as you discover and understand the lesson prepared for you. Pre-tests as provided to measure your prior knowledge on lessons in each SLM. This will tell you if you need to proceed with completing this module or if you need to ask your facilitator or your teacher’s assistance for a better understanding of the lesson. At the end of each module, you need to answer the post-test to self-check your learning. Answer keys are provided for each activity and test. We trust that you will be honest in using these. In addition to the material in the main text, Notes to the Teacher are also provided to our facilitator and parents for strategies and reminders on how they can best help on your home-based learning. Please use this module with care. Do not put unnecessary marks on any part of this SLM. Use a separate sheet of paper in answering the exercises and tests. And read the instructions carefully before performing each task. If you have any questions in using this SLM or any difficulty in answering the tasks in this module, do not hesitate to consult your teacher or facilitator. Thank you. 2 What I Need to Know This module was designed and written with you in mind. It is here to help you master the nature of Chemistry. The scope of this module permits it to be used in many different learning situations. The language used recognizes the diverse vocabulary level of students. The lessons are arranged to follow the standard sequence of the course. But the order in which you read them can be changed to correspond with the textbook you are now using. This module is divided into two lessons namely: ● Lesson 1- Chemical Change ● Lesson 2- Chemical Reactions After going through this module, you are expected to: a. b. c. d. identify chemical change; classify reactions according to the different types; write chemical equations; and apply the principles of conservation of mass to chemical reactions (S10MT-IVe-g-23). What I Know Directions. Choose at least 10 elements or compounds from the list given below. Then, write the symbol or formula of your chosen elements or compounds. Write your answers in your notebook/on a separate sheet of paper. 1. Water 11. Copper 2. Table salt 12. Baking Soda 3. Oxygen 13. Toothpaste 4. Sugar 14. Mouth wash 5. Iron 15. Carbon 6. Hydrogen 16. Vinegar 7. Aluminum 17. Nitrogen 8. Carbon Dioxide 18. Calcium 9. Sodium 19. Magnesium 10. Gold 20. Helium 3 Lesson 1 Chemical Change Change is the only permanent thing in the world. Changes in matter can either be physical or chemical. Physical change occurs when the appearance of an object is altered without forming a new type of substance. Chemical change involves a transformation of one substance into a new type by altering its chemical composition. In your Grade 9 Science, you have learned that chemical bonding includes breaking up of bonds and the formation of new bonds. When new bonds are formed, new substances are also produced. These processes of bond breakage and formation cause atoms to rearrange themselves brought chemical changes in matter. What’s In Activity 1 Directions. Classify the given processes into physical change or chemical change. Write your answers in your notebook/ on a separate sheet of paper. 1. Burning of paper 2. Washing of plates 3. Leaves in a compost pit 4. Ripening of fruits 5. Leftover foods in the trash bins 4 What’s New Activity 2: Exploring the Evidences of Chemical Reactions Directions: Perform the simple experiment below. Write your answers in your notebook/ on a separate sheet of paper. Objective: Exploring chemical reactions Materials Needed: bleaching reagent (chlorox) colored paper mentos candy (5 pcs) matchsticks piece of paper soft drinks (15 mL) Procedures: 1. Place a piece of colored paper in a pan then pour a drop of bleaching agent (chlorox). Observe what happens. 2. Open the soft drinks then put the mentos inside the bottle. Observe what happens. 3. Get a piece of paper and lift it up using the matchstick. Observe. Guide Questions: 1. What happened to the colored paper? 2. What is formed in coke after putting the mentos? 3. What is formed when you lift the paper? What is It Evidences of Chemical Reactions Chemical change is caused by chemical reactions. Though reactions occur at microscopic level there are still some observations or physical changes which can be used as indicators that chemical reactions are happening. There are things that will help us to identify if a certain substance undergoes chemical reactions and we call it evidences of chemical reactions. The evidences are production of light, evolution of gas, temperature change, change in intrinsic properties (color, odor) and formation of precipitate. Chemical reactions are observed with the combination of the 5 evidences. Fire was the greatest discovery of all time. The earliest people considered it as one of the earliest elements. George Ernst Stahl stated that when a material burns, it releases a substance known as phlogiston and he called it the Phlogiston Theory. Antoine Lavoisier contradicts this theory as he carefully observed in his experiment that instead of releasing 5 a substance, the material being burned reacts with oxygen. This is now known as the Theory of Oxidation, and this is accepted up to this day. There are 3 factors that should be present in proper conditions and proportions for burning to occur: 1. Fuel 2. Oxygen 3. Heat What’s More Activity 3 Directions: Identify the evidences that occurred in the given chemical change. Choose your answers inside the box and write them in your notebook/ on a separate sheet of paper. Production of light Evolution of gas Change in intrinsic properties 1. 2. 3. 4. Temperature change Formation of precipitate burning of paper leftover foods in the trash bins dead rat ripening of mango 6 What I Have Learned 1. When a physical change occurs there is no breaking and forming of bonds. There are certain things that will help us identify if a chemical reaction has taken place. We call these evidences of chemical reactions. These are production of light, evolution of gas, temperature change, color change, and formation of precipitate. 2. Three factors that should be present in proper conditions and proportions for burning to occur: fuel, oxygen, & heat What I Can Do Activity 3. Recreate Me Objective: Apply the evidences of chemical reactions Materials: Black t-shirt bleach (cholorox) double-sided tape Procedures: 1. Place the t-shirt on a flat surface. 2. Use double-sided tape to form your desired design. 3. Pour on the bleach on the t-shirt. 4. Let it dry. 7 Lesson 2 Chemical Reactions Chemical reactions create new substances. Substances that undergo chemical changes is called reactant while the new type of substances formed is product. Chemical equation describes chemical reactions by identifying the reactants and products. It also uses symbols and formulas of substances involved in the chemical reaction. What’s In Activity 4 Directions. Group the following ingredients used in cooking sopas, pork adobo and burger. Write your answers in your notebook/on a separate sheet of paper. a bun pork macaroni margarine tomatoes onion burger patty black pepper soy sauce garlic carrots onion evaporated milk chicken cabbage hotdog garlic cheese lettuce vinegar sopas pork adobo 8 burger What is It Chemical equation shows the symbols or formulas of the reactants and products, the phases (solid, liquid, gas) of these substances, and the ratio of the substances as they react. In writing chemical equations, you should know the common symbols needed in writing equations. Symbols + → (s), (l), (g), (aq) HEAT Pt Example of chemical equation: 2 𝐻2(𝑔) + 𝑂2(𝑔) Reactants Meaning Shows a combination of reactants or products To produce; to form; to yield (s)-solid (l)-liquid (g)-gas (aq)aqueous (substance is dissolved in water) Reversible reaction Indicates that heat is supplied to the reaction A formula written above or below the yield sign indicates its use as a catalyst or solvent 2 𝐻2 𝑂 Product There are different types of chemical reactions: combination, decomposition, single displacement, double displacement, combustion, and acid-base reaction. Different Types of Chemical Reactions COMBINATION OR SYNTHESIS REACTION When 2 or more reactants combine to form a single product A + B → AB DECOMPOSITION REACTION A single reactant breaks down into simpler ones. (2 or more products) AB → A+B SINGLE DISPLACEMENT or Replacement REACTION When one element replaces another element from a compound. The more active element takes the place of the less active element in a compound. A + BC → AC + B 9 DOUBLE DISPLACEMENT REACTION (Metathesis) When the positive ions (cations) and negative ions (anions) of different compounds switch places, forming two entirely different compounds AB + CD → AD + CB COMBUSTION (Burning) REACTION When oxygen combines with a hydrocarbon (compound containing hydrogen and carbon) to form a water and carbon dioxide. Example of which is the burning of butane gas. CH4 + O2 → 𝐶𝑂2 + 𝐻2 𝑂 𝑐𝐻4 𝐻10 + 𝑂2 ACID-BASE REACTION A special kind of double displacement reaction that takes place when an acid and base react with each other. The H+ of the acid reacts with the OH- of the base forming water. The other product is salt. HCl + NaOH → NaCl + H2O Activity 5 Directions. Classify the following unbalanced chemical equations according to the six types of chemical reactions. Write your answers in your notebook/ on a separate sheet of paper. 1. AgNO3 + Cu → Cu(NO3)2 + Ag 2. P4 + O2 → P2O5 3. HBr + Al(OH)3 → AlBr3 + H2O 4. O3 → O- + O2 5. C3H6O + O2 → CO2 + H2O 6. HBr + KOH → KBr + H2O 7. H2 + N2 → NH3 8. Hg2O → Hg + O2 9. Cl2 + NaBr → NaCl + Br 10. CH4 + O2 → CO2 + H2O 11. SO2 + O2 → SO3 12. HgO → Hg + O2 13. P + O2 → P4O10 14. KClO4 → KCl + O2 15. Fe2O3 + C → Fe + CO2 16. H2 + AgNO3 → Ag + HNO3 17. CaCO3 + HCl → CaCl2 + H2CO3 18. Cu + AgNO3 → Ag + Cu(NO3)2 19. Mg + O2 → MgO + energy 20. HCl + NaOH → NaCl + H2O 10 During a chemical reaction, matter is neither created nor destroyed. Atoms of substances undergoing reactions simply rearrange themselves thus matter is said to be conserved. This concept is known as the Law of Conservation of Mass. Activity 6: Popcorn Mass Directions. Answer the questions given below based on your observations. Do this in your notebook/on a separate sheet of paper. Note to the students and parents/guardian: Ask the help of your guardian/ parent in performing this activity. Objective: Perform an activity that illustrates Law of Conservation of Mass Materials: Popcorn kernel (5 grams) Oil Weighing scale Frying Pan Procedures: 1. Weigh the popcorn kernels and the oil that you will be using. 2. Cook the popcorn kernels in the pan. Be careful in using the stove. 3. Weigh the cooked popcorns. Guide Question: What is the difference between the weight of the uncooked popcorns and the cooked popcorns? Balancing Equation Balancing chemical equations is done to obey the Law of Conservation of Mass. Balanced equations depict that the number of atoms on the reactant side is equal to the number of atoms on the product side. Generally, making the atoms at both sides of the equation is done by putting coefficients before the symbol or formula of the substances present in the equation. To balance a chemical equation, we have to perform the following steps: 1. Rewrite the unbalanced chemical equation. Example: Al + O2 → Al2O3 2. Make a list of all the elements on the reactants (left) and products (right). Reactants Products Al: Al: O: O: 3. Identify the atoms in each element. Reactants Products Al: 1 Al: 2 O: 2 O: 3 11 4. Multiply the numbers of atoms. Observe the element whose atom is not equal. Reactants Products Al: 1 ∙ 2 = 2 Al: 2 O: 2 ∙ 3 = 6 O: 3 ∙ 2 = 6 5. Place coefficient in front of the equation. 2 Al + 3 O2 → 2 Al2O3 Reactants Products Al: 1 ∙ 2 = 2 Al: 2 O: 2 ∙ 3 = 6 O: 3 ∙ 2 = 6 6. Check your equation. If it is not balanced rework your multiplication. 4 Al + 3 O2 → 2 Al2O3 Reactants Products Al: 1 ∙ 2 ∙ 2 = 4 Al: 2∙ 2 = 4 O: 2 ∙3 = 6 O: 3 ∙ 2 = 6 7. Balance chemical equation 4 Al + 3 O2 → 2 Al2O3 Reactants Products Al: 4 Al: 4 O: 6 O: 6 What’s More Activity 7 Directions. Choose 5 equations and balance them by following the appropriate steps. Write your answers in your notebook/ on a separate sheet of paper. 1. Fe + O2 → Fe2O3 2. H2 + Cl2 → HCl 3. Ag + H2S → Ag2S + H2 4. CH4 + O2 → CO2 + H2O 5. HgO → Hg + O2 6. Cu + AgNO3 → Ag + Cu(NO3)2 7. SO2 + O2 → SO3 8. CaCO3 + HCl → CaCl2 + H2CO3 9. P + O2 → P4O10 10. KClO4 → KCl + O2 12 What I Have Learned 1. A chemical equation is a chemist’s shorthand for a chemical reaction. It shows the symbols or formulas of the reactants and products, the phases (solid, liquid, gas) of these substances and the ratio of the substances as they react. 2. Chemical reactions are classified according to the following types: combination: A + B → AB decomposition: AB → A + B single displacement: A + BC → AC + B double displacement: AB + CD → AD + CB combustion (reaction with oxygen-producing carbon dioxide and water) acid-base: reaction between acid and base 3. Law of Conservation of Mass states that during physical and chemical changes, matter cannot be created nor destroyed; the mass of the reactants is equal to the mass of products. What I Can Do Activity 8. Directions: Decode the message below by matching the balanced equation of column A to column B. Write your answers and solutions in your notebook/on a separate sheet of paper. Column A 1. Co + H2O → Co2O3 + H2 2. Fe+ Cl2 → FeCl3 3. Al + CuCl2 → AlCl3 + Cu 4. Fe+ O2 → Fe2O3 5. FeBr3+ H2SO4 → Fe2(SO4)3+ HBr 6. C4H6O3+ H2O → C2H4O2 7. C2H4+ O2 → CO2+ H2O 8. C4H10O+ O2 → CO2+ H2O 9. C7H16 + O2 → CO2+ H2O 10. H2SiCl2+ H2O → H8Si4O4+ HCl Column B A. 4 H2SiCl2+4 H2O →H8Si4O4+8 HCl C. C2H4+3 O2 → 2 CO2+2 H2O E. 2 FeBr3+3 H2SO4 → Fe2(SO4)3+6 HBr H. 2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu I. C4H6O3+H2O→2 C2H4O2 M. C7H16+11 O2 →7 CO2+8 H2O R. 2 Fe+3 Cl2 → 2 FeCl3 S. 4 Fe+3 O2 → 2 Fe2O3 T. 2 Co + 3 H2O → Co2O3 + 3 H2 Y. C4H10O+6 O2 → 4 CO2+5 H2O ___ ___ ___ ___ ___ ___ ___ ___ ___ 7 3 5 9 6 4 1 2 8 13 ___ ___ 6 4 ___ ___ ___ ___ 5 10 4 8 Assessment Multiple Choice. Directions. Read each item carefully. Choose the letter of the best answer and write it on your notebook/on a separate sheet of paper. 1. Which of a. b. c. d. the following is not an evidence of chemical reactions? Evolution of Gas Production of light Same substance Temperature change 2. Who discovered the Phlogiston theory? a. Amadeo Avogadro b. Antoine Lavoiser c. Charles Darwin d. George Ernst Stahl 3. Who is the proponent of the Oxidation theory? a. Amadeo Avogadro b. Antoine Lavoiser c. Charles Darwin d. George Ernst Stahl 4. For burning to occur, the following factors should be present in proper conditions and proportions. I. Fuel II. Oxygen III Heat IV.Nitrogen a. I, II, III b. I, II, IV I, III, IV c. II, III, IV 5. How does a reactant differ from a product? a. Reactants are the entering material. b. Products are the resulting substances. c. Both a and b d. none of a, b c 6. A + BC a. b. c. d. → AC + B is an equation used in __________________. Combination reaction Decomposition Reaction Double displacement reaction Single displacement reaction 7. NAOH + a. b. c. d. KNO3 → NaNO2 + KOH is the formula used in __________________. Combination reaction Decomposition Reaction Double displacement reaction Single displacement reaction 14 8. How many atoms of Zn, H, Cl are present in the reactants below? Zn + 2 HCl → ZnCl2 + H2 a. 1;1;1 b. 1;2;2 c. 2;2;2 d. none of a,b 9. It is the law which states that the total mass of reactant is equal to the total mass of the product. No new atoms are created nor destroyed. a. Law of Conservation of Energy b. Law of Conservation of Mass c. Law of Conservation of Momentum d. Law of Conservation of Weight 10. It is an equation that uses chemical symbols and formulas to represent a chemical reaction. a. Chemical equation b. Nuclear equation c. Nuclear reaction d. Mathematical equation Additional Activities Poster Making Directions. In a short bond paper, draw one scenario inside your house that undergoes chemical reactions. Illustrate first the original setting then identify what happens during reactions and draw the product or the output. Rubrics for Scoring Category Sketch Understanding the topic 5 pts The sketch is neat, and necessary markings are found on the paper. It shows a clear and legible concept. The learner understood the lesson and can apply it to the activity. 4 pts The sketch is legible. The concept is clear and legible but can neater 3pts The sketch is somewhat okay but is not exactly neat and legible. 1pt The sketch is not legible or clear at all. The learner has sufficient comprehensio n of the lesson and can apply it to the activity. The learner has sufficient understanding of the lesson but is not applied in the activity. The learner has not understood most of the lesson & cannot apply it to the activity. Total: (10 pts) 15 Score What’s 1. 2. 3. 4. 5. In Chemical change Physical change Chemical change Chemical change Chemical change What’s New Q1. Answers may vary Q2. Answers may vary Q3. Answers may vary Activity 4 Burger a bun, burger patty, tomato, cheese, lettuce Pork Adobo onion, garlic, pork, soy sauce, vinegar, black pepper Sopas macaroni, evaporated milk, onion, garlic, chicken, hotdog, carrots, cabbage, margarine 16 Activity 5 1. Single Displacement 2. Synthesis 3. Double Displacement 4. Decomposition 5. Combustion 6. Acid-base 7. Synthesis 8. Decomposition 9. Single Displacement 10. Combustion 11. Combustion 12. Decomposition 13. Combustion 14. Decomposition 15. Single Displacement 16. Single Displacement 17. Double Displacement 18. Single Displacement 19. Combustion 20. Acid-base Activity 6 Equal Activity 7 1. 4 Fe + 3 O2 → 2 Fe2O3 2. H2 + Cl2 → 2 HCl 3. 2 Ag + H2S → Ag2S + H2 4. CH4 + 2 O2 → CO2 + 2 H2O 5. 2 HgO → 2 Hg + O2 6. Cu + 2AgNO3 → 2Ag + Cu(NO3)2 7. 2 SO3 + O2 → 2 SO3 8. CaCO3 + 2 HCl → CaCl2 + H2CO3 9. 4P + 5O2 → P4O10 10. KClO4 → KCl + 2O2 Activity 8 CHEMISTRY IS EASY Assessment 1.c 2.d 3.b 4.c 5.c 6.d 7.c 8.b 9.b 10.a Answer Key References A. Book Acosta, Herma D. et. al (2015). Science Learner’S Manual. 1st edition. Rex Bookstore, Inc., 5th Floor Mabini Building Deped Complex Meralco Avenue, Pasig City. B. Websites https://chem.liretexts.org/Courses/University_of_Arkansas_Little_Rock/chem_1 402%3A_General_chemistry_1_(belford)/Text/3%3A_chemical_reactions/3.1%A_I ntoduction_to_chemial_Equations https://www.nationalgeographic.org/article/conservation-matter-during-physical-and chemical-changes/6th-grade/ https://terpconnect.umd.edu/-wbreslyn/chemistry/reactions/inde.html https://www.google.com/amp/s/www.instructales.om/how-to-alane-a-chemicalequations-1/%3famp_page=true 17 For inquiries or feedback, please write or call: Department of Education - Bureau of Learning Resources (DepEd-BLR) Ground Floor, Bonifacio Bldg., DepEd Complex Meralco Avenue, Pasig City, Philippines 1600 Telefax: (632) 8634-1072; 8634-1054; 8631-4985 Email Address: blr.lrqad@deped.gov.ph * blr.lrpd@deped.gov.ph