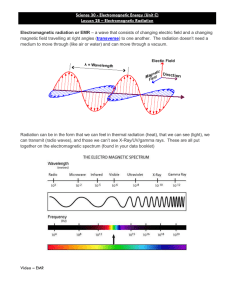
Announcements - Purchase the Digital copy of the book, not access code ◦ Access code is back-ordered - Blank digital copies of in-class handouts available on Moodle ◦ If you like to annotate on tablets, or can use for studying before exams - Office hours, today, 1 – 2:30 pm, SCI 284 - Alchemist Session, Thurs., 7 - 9 pm, SCI 128 - Review Sessions for Primer to Chem 10: Friday 2 – 3:30 pm, SCI 183 Assignments - P. Set 1 and OWL HW 1- due Wed., 9/8 at noon Today - Electromagnetic radiation (6.1) - Energy Quantization (6.2) Chapter 6: Structure of Atoms Sections 6.1-6.2 Electromagnetic radiation 1864, James Maxwell ◦ light and radiation = oscillating, wave-like electric and magnetic fields ◦ Visible light, microwaves, x-rays, etc = Electromagnetic radiation EMR like behavior and travels through space the speed of at light in radiant 1 É the wave position exhibits wave vacuum I f energy that Wavelength (λ, units = m): distance between a given point on a wave and the corresponding point in the next cycle of the wave Frequency (ν units = s-1s, Hz): the number of waves (oscillations) that pass a given point in some unit of time (usually seconds) Speed of light speed of light X vacuum in C M C X t 2 998 108M x x s z Electromagnetic spectrum Decrease frequency, increase wavelength, and vice versa Xx Xv m a Problem 1 An FM radio station broadcasts at 99.5 MHz. Calculate the wavelength of the corresponding radio waves. N 99.5 V 99.5 C 2.998 MHZ 106 106 HE Hz D XXV 108 M S X X 3.070 7 99.8 106 St Radiation discoveries Pre-1900, Classical Physics ◦ Thought matter and energy were distinct F consists of particles has mass pecified E E light a is wave massless young experimental cassical theory 1 p ◦ Thought matter can absorb or emit any quantity of energy energy iscontinuous match didn't theory experimental observations in ur or catastrophe region Muy Quantization of energy 1900, Max Planck ◦ Accounted for observed profiles by postulating that energy is quantized ◦ Quantization: emitted energy can only have certain energies hut integer frequency constant ◦ Discrete units, or “packets” of energy = quanta change leg energy it's heated in as Ann DE it al between change adjacent E levels of system a e f Joules n w Planks constant3 6 626 10 5 Problem 2 The blue color in fireworks is often achieved by heating copper(I) chloride (CuCl) to about 1200 degrees Celsius. The hot compound emits blue light at a wavelength of 450 nm. What is the energy emitted by CuCl? T 12000C c XXV 950 nm 9 2 2.998 108 Mls 450 10 M XV 159 6.66 X 10M s V E E W 6.626 10 34 J's x 6.66 101454 104950 5 4.4 450 E C V E X V her M Photoelectric effect 1905, Albert Einstein – photoelectric effect ◦ Bombarded a piece of metal with light ◦ Electrons emitted only when frequency of light was greater than or equal to threshold frequency →Proposed that electromagnetic radiation is quantized →EMR can be viewed as a stream of particles called photons A EMR has ooo ooo particledality wave light light Ephoton as as wave a a Net Maxwell Planck stream of photons Einstein