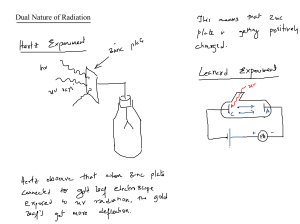
Section 1 (Page 136) 1. Describe two ways Rutherford's was limited in totally describing the atom. 2. In the early 1900s scientist began to unravel the puzzle of what? _____________________________________________________________________________ _____ 3. Define electromagnetic radiation. _____________________________________________________________________________ 4. Write the formula for Electromagnetic Wave Relationship and describing each component. 5. What is the speed of light in a vacuum?____________________________________________ 6. Draw a wave with 4 wavelengths then label, trough, origin, wavelength, crest, and amplitude 7. If a wave of light travels at the same speed how is red light differ from blue light? 8. What are the 7 wave frequencies of the electromagnetic spectrum from lowest to highest? 9. List 4 human activities that produce radiation. a) b) c) d) 10. What does temperature have to do with the “quantum concept”? 11. What did Max Planck conclude about heated objects? 12. What is a quantum? 13. Planck proposed that energy emitted by hot objects was _____________________. 14. Write the Energy of Quantum formula below and label each component. 15. What is the value of Planck's constant? _________________________________ 16. The equation shows what about energy of radiation _______________________ as the radiation's _______________________ increases. 17. Matter can emit or absorb ____________________________ in whole number increments of whole numbers. 18. What is the photoelectric effect? 19. A metal _________________ ____________ eject photoelectrons below a specific frequency of ________________________ light. 20. Draw the photoelectric effect in figure 7 below 21. What did Albert Einstein propose about light?(2 things) 22. Write the formula for the energy of Photon. 23. Complete Practice problem # 1 (a-c), show work for each a.. b. c. 24. How does a neon light work? 25. An element has a set of frequencies of electromagnetic waves emitted by the atoms is what? 26. Can an atomic emission spectrum be used to identify an element? ____________________ Section 2 (page 146)