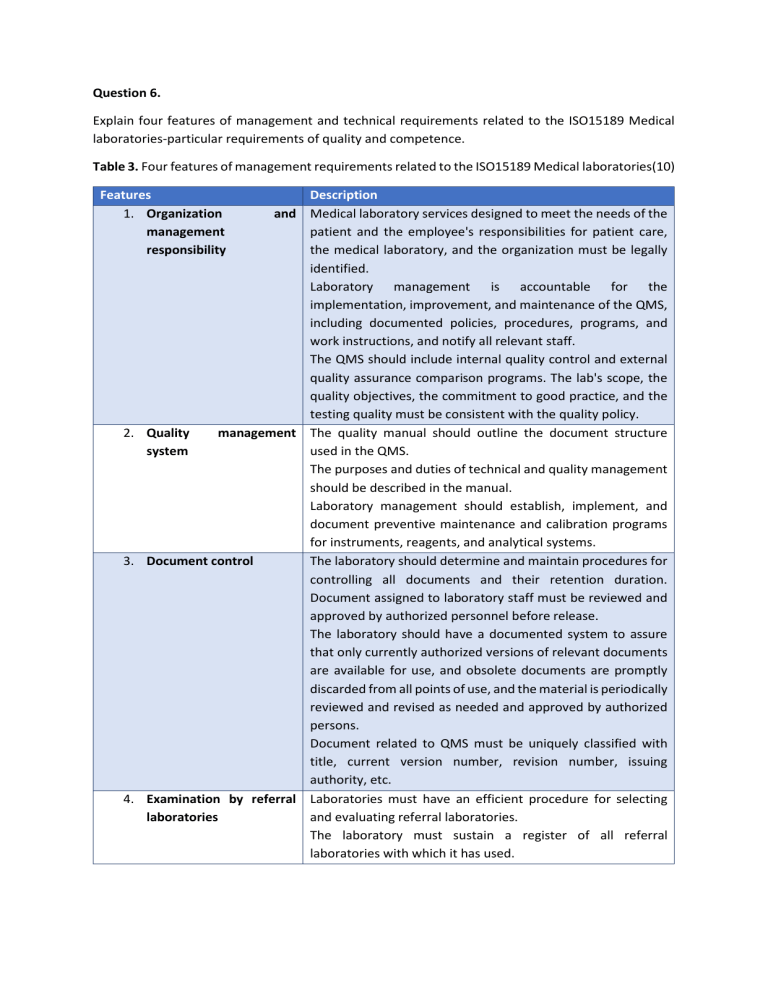
Question 6. Explain four features of management and technical requirements related to the ISO15189 Medical laboratories-particular requirements of quality and competence. Table 3. Four features of management requirements related to the ISO15189 Medical laboratories(10) Features 1. Organization management responsibility Description and Medical laboratory services designed to meet the needs of the patient and the employee's responsibilities for patient care, the medical laboratory, and the organization must be legally identified. Laboratory management is accountable for the implementation, improvement, and maintenance of the QMS, including documented policies, procedures, programs, and work instructions, and notify all relevant staff. The QMS should include internal quality control and external quality assurance comparison programs. The lab's scope, the quality objectives, the commitment to good practice, and the testing quality must be consistent with the quality policy. 2. Quality management The quality manual should outline the document structure system used in the QMS. The purposes and duties of technical and quality management should be described in the manual. Laboratory management should establish, implement, and document preventive maintenance and calibration programs for instruments, reagents, and analytical systems. 3. Document control The laboratory should determine and maintain procedures for controlling all documents and their retention duration. Document assigned to laboratory staff must be reviewed and approved by authorized personnel before release. The laboratory should have a documented system to assure that only currently authorized versions of relevant documents are available for use, and obsolete documents are promptly discarded from all points of use, and the material is periodically reviewed and revised as needed and approved by authorized persons. Document related to QMS must be uniquely classified with title, current version number, revision number, issuing authority, etc. 4. Examination by referral Laboratories must have an efficient procedure for selecting laboratories and evaluating referral laboratories. The laboratory must sustain a register of all referral laboratories with which it has used. The referring laboratory, not the referral laboratory, should be accountable for assuring that test results are provided to the requestor. Table 4. Four features of technical requirements related to the ISO15189 Medical laboratories (10). Features 1. Personnel Description Laboratory personnel are competent by determining whether having appropriate education, training, skills, and experience. Personnel must be provided with required training and are evaluated for the effectiveness of the training to meet the quality objectives of the organization. Documentation of employee qualifications, membership of professional associations, training and performance evaluation records in the laboratory should be provided as part of the NATA competency assessment process. Proof of acceptance and recognition of overseas qualifications must be available 2. Accommodation and Laboratory and office facilities shall provide a proper working environmental environment for the tasks to be carried out to meet the conditions requirements of customer and the quality management system. This includes storage facilities, supporting services, patient sample collection equipment and supplies, maintenance of facilities, and environmental circumstances. The laboratory shall have procedures in place to control and document environmental conditions that do not adversely affect the performance of sampling equipment and equipment. The sterility, dust, humidity, temperature according to relevant technical activities need special attention. 3. Laboratory equipment, The laboratory shall have all necessary equipment to provide reagents, and its services. Laboratories must also have a procedure that consumables describes how they select equipment. Equipment includes documented procedures, proficiency testing, manuals, measurement calibration and traceability, maintenance and repair, adverse indentation reports and records. Reagents and consumables include documented procedures and instructions, reception and storage, standards and materials reference, inventory management, adverse incident reports and records. 4. Post-examination Authorized personnel systematically review test results, processes authorized assess them against the patient's clinical information before releasing the results. Retention of primary and other laboratory samples is subject to the approved policy. Handling samples should be carried out according to local regulations or recommendations for waste management and in accordance with internal policy.




