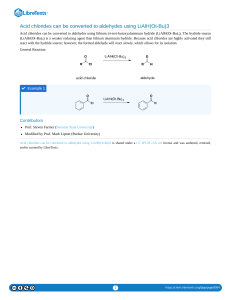
KABARAK UNIVERSITY SCHOOL OF SCIENCE, ENGINEERING & TECHNOLOGY DEPARTMENT OF PHYSICAL & BIOLOGICAL SCIENCES COURSE CODE : COURSE TITTLE SCPH 2121 : ORGANIC CHEMISTRY III E-LEARNING MAY-AUGUST2020 SEMESTER AUTHOR: Dr. KEBENEI J. SELLAH 1 REFERENCE BOOKS 1. Bruice P.Y,.(2001).Organic Chemistry (3rd Edition), Prentice Hall, 2. Solomons T.W.G, (1997) Organic Chemistry (6th Edition), John Wiley and sons Inc.. 3. Sandler, S.R. and Karo, W., (1992.) A source book of advanced organic laboratory preparations, Academic Press Inc. 4. Morrison, R.T. and Boyd, R.N (2001). Organic Chemistry, (6th Edition), Prentice Hall 2 1.0 INTRODUCTION Carbonyl compounds are compounds that consist of carbon double bonded oxygen (C=O) group in their molecules. C=O group is called carbonyl group. Families of compounds that consist of the carbonyl group are as follows: Aldehydes – R-CHO Ketones – R2CO Carboxylic acid - RCOOH Esters – R-COOR’ Acid Chlorides – R-COCl Acid anhydride – (RCO)2O Amides – RCONH2 Carbonyls are classified into two categories: Class I carbonyls – are carbonyl compounds which have a group of atoms that can be replaced by a nucleophile. This are esters, acid chlorides, Acid anhydride and amides. Class II carbonyls – carbonyl compound that consist of group of atoms that cannot be replaced by a nucleophile. These are aldehydes and ketones due to the H – atom and alkyl groups cannot be substituted. 1.1 LAB PREPARATIONOF ALDEHYDES 1. By oxidation of primary alcohols (RCH2OH) Primary alcohols are oxidized to aldehydes when reacted with acidified KMno4 or K2Cr2O7. Therefore KMno4/H+ is decolorized and K2Cr2O7/H+ change from orange to green solution. Note: excess of the alcohol is used to stop further oxidation of the aldehyde into carboxylic acid. 2. Oxidation of methylbenzene (Prep of Benzaldehyde) 3 Methylbenzene is reacted with Cl2 gas and heated to form benzyl dichloride followed by hydrolysis to form benzaldehyde. 3. By reduction of acid chlorides Acid chlorides are organic acid that consist a carbonyl group (C=O) and the carbonyl carbon atom is bonded with one alkyl group and a chloride atom. They have general formula RCClO Structure: , example ethanoylchloride - Therefore acid chlorides are reduced to aldehydes by reacting with strong reducing agent example: (a). Lithium Aluminium hydride (LiAlH4) in a base (NaOH). Example OR (b).Using strong reducing agent like Lithium tri-tert-butoxy alluminium hydride (LiAlH(O-t-Bu)3 in n-hexane at -780C of temperature followed by hydrolysis. Structure of LiALH(O-t-Bu)3 4 General equation Example: 3. By reduction of Esters (RCOOR’) Esters react with strong reducing agent DiisobutylAluminium Hydride in hexane solvent at a temperature of -780C followed by hydrolysis to form an aldehyde. Structure of DIBAl-H 5 General equation Example 4. By reduction Alkylnitriles (Cyanoalkane – RCN) Alkynitriles (cyanoalkanes) react strong reducing agent DIBAl-H in hexane at -780C followed by hydrolysis to give an aldehyde. General equation Example 1.12 LAB PREPARATIONOF KETONES (R2C=O) 1. By oxidation of secondary alcohols Secondary alcohols are oxidized to ketones by acidified KMno4 or K2Cr2O7. Therefore KMno4/H+ is decolorized and K2Cr2O7/H+ change from orange to green solution. 6 2 By reaction of acid chloride/bromide with benzene Acid chloride/bromide reacts with benzene in presence of a Lewis acid to form a ketone. General equation Example 3. From Lithium Dialkyl cuprate (R2CuLi) Acid chloride reacts with lithium dialky cuprate in ether solvent at -78 0C of temperature to form 7 ketone. General equation Example 4. From alkyl nitriles (Cyanoalkane –RCN) (i). Cyanoalkanes (RCN) reacts with Grignard reagent (RMgX) in ether solvent followed by hydrolysis to yield Ketones. Gerneral equation Example 8 (ii). Also cyanoalkanes react with alkyl lithium (R-Li) in ether solvent followed by hydrolysis to form ketones. General equation Example 5. Ketones from alkynes Alkynes react with water in presence of a catalyst Hg2SO4/H2SO4 to form an intermediate called alkenol (enol) which undergo rearrangement to form a ketone. Enols consist a c=c-bond and OH group is bonded to the double bond C-atom (R-CH=CH(OH)R’). That is –en is from alkene and –ol is from alcohol. General equation The rearrangement is called keto-enol tautomerization. Example 9 Mechanism of Tautomerization 10