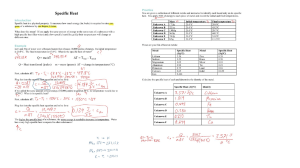
Modeling Energy Changes Student Guide Assignment Summary In this assignment, you will write and balance a chemical equation. Then, you will use a table of enthalpy values to calculate the energy change in the reaction. Next, you will create a model of the energy change in the reaction. Finally, you will write an explanation that describes the energy change in the reaction. Background Information Chemical bonds contain potential energy. The breaking and forming of bonds that occurs during a chemical reaction results in the release or absorption of energy. Chemical reactions that release energy into the surrounding environment are called exothermic reactions, while chemical reactions that absorb energy from the surrounding environment are called endothermic reactions. Since energy is conserved during chemical reactions, the total amount of energy in the reaction and the surrounding environment remains the same. During a chemical reaction, bonds in the reactants are broken and new bonds are formed in the products. Energy is needed to break the bonds in the reactants, so breaking bonds is an endothermic process. Forming new bonds, on the other hand, is an exothermic process because energy is released when new bonds are formed in the products. A chemical reaction is an exothermic reaction if more energy is released when new bonds are formed in the products than is used to break the bonds in the reactants. Conversely, if more energy is used to break the bonds in the reactants than is released when new bonds are formed in the products, the chemical reaction is an endothermic reaction. The amount of energy change in a reaction can be determined by calculating the enthalpy of reaction (∆Hrxn). If the enthalpy of reaction is negative, then the reaction is exothermic. If the enthalpy of reaction is positive, then the reaction is endothermic. Energy graphs are used to represent energy changes within a reaction. These graphs show the amount of energy in the reactants and products so one can easily see the change in energy that occurs during a chemical reaction. This change in energy is the energy that is either absorbed or released during the chemical reaction. Materials Student worksheet Blank sheet of paper Drawing/writing utensil Assignment Instructions Step 1: Prepare for the project. a) Read the entire Student Guide before you begin this project. b) If anything is not clear to you, be sure to ask your teacher for assistance before you begin. c) Gather the materials you will need to complete this project. Copyright © Edgenuity Inc. Student Guide (continued) Step 2: Write and balance the chemical equation. a) Read the word equation: “Propane gas plus oxygen gas produces __________.” b) Convert the word equation to a chemical equation and complete the reaction. Be sure to balance the chemical equation. c) Record the balanced chemical equation on the Student Worksheet. Step 3: Determine the amount of energy change in the reaction. a) Use the table of enthalpy values (Table A) provided in the Student Worksheet to locate the enthalpy of formation (∆Hf) for each reactant and each product. Record these values along with the reactants and products in Table B of the Student Worksheet. b) Determine the total enthalpy of the reactants and the total enthalpy of the products. Record these values in Table C of the Student Worksheet. c) Use the following formula to find the net change in enthalpy for the reaction and to determine whether the reaction is endothermic or exothermic. Hrxn H f, products H f, reactants Record your answers in Table D. Step 4: Model the energy change in the reaction. a) Create an energy graph that illustrates the energy change in the reaction. b) Construct your graph on a blank sheet of paper. Be sure to label the axes, provide a title, and identify the reactants and products on the graph. Step 5: Explain the energy change in the reaction. a) Create a new blank document. Type your name at the top. b) Write a few paragraphs describing the chemical reaction and explaining the energy change in the reaction. Your document should: i. identify the reactants and products. ii. describe the change in energy that occurs as bonds are broken and formed. iii. identify how the potential energy of the reactants compares to the potential energy of the products. iv. state and explain the net change in enthalpy. v. identify whether the reaction is endothermic or exothermic. vi. explain how energy is conserved between the reaction and the surrounding environment. Step 6: Evaluate your project using this checklist. If you can check each criterion below, you are ready to submit your project. Did you write and balance the chemical equation on the Student Worksheet? Did you determine the amount of energy change in the reaction? Be sure that you have completed Tables B, C, and D on the Student Worksheet. Copyright © Edgenuity Inc. Student Guide (continued) Did you create a model of the energy change in the reaction? Your model should be an energy graph that clearly shows the change in energy for the chemical reaction. Be sure that the axes are labeled, a title is provided, and the reactants and products have been identified on the graph. Did you describe the chemical reaction and explain the energy change in the reaction? Your explanation should identify the reactants and products, describe the change in energy that occurs as bonds are broken and formed, identify how the potential energy of the reactants compares to the potential energy of the products, state and explain the net change in enthalpy, identify whether the reaction is endothermic or exothermic, and explain how energy is conserved between the reaction and the surrounding environment. Step 7: Revise and submit your project. a) If you were unable to check off all of the requirements on the checklist, go back and make sure that your project is complete. b) When you have completed your project, submit your Student Worksheet and energy graph to your teacher for grading. c) Submit your typewritten document through the virtual classroom. Be sure your name is on it. Step 8: Clean up your work space. a) Clean up your work space and throw away any trash. b) Congratulations! You have completed your project. Copyright © Edgenuity Inc. Student Guide (continued) Student Worksheet: Modeling Energy Changes Balanced Chemical Reaction Enthalpy of Formation Table A: Enthalpy of Formation Values for Various Substances Substance Hf (kJ/mol) NH3(g) –46.11 NO2(g) +33.18 CH4(g) –74.81 C2H4(g) +52.26 C2H6(g) –84.68 C3H8(g) –103.85 C3H6(g) +20.42 CO2(g) –393.51 H2O(g) –241.82 CH3OH(l) –238.86 Table B: Enthalpy of Formation of Reactants and Products Substance Hf (kJ/mol) Copyright © Edgenuity Inc. Student Guide (continued) Table C: Total Enthalpy of Formation of Reactants and Products Hf (kJ/mol) Total enthalpy of reactants Total enthalpy of products Enthalpy of Reaction Table D: Enthalpy of Reaction Enthalpy of reaction Hrxn(kJ/mol) Reaction – endothermic or exothermic Copyright © Edgenuity Inc.