Testes de Íons Positivos e Negativos: Teste de Chama e Precipitação
advertisement

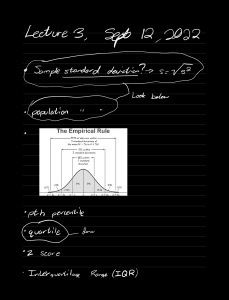
Positive and Negative ion tests Test ① for positive ions flame test ' ' : : Method q.oq.ge cations 1- results : clean nichrome nichrome wire wire 1¥ @ ;É% . Dip 2- 3- Hold sample in > you use to have blue a 1. nichrome wire in Roaring flame ② Precipitation Method ' because Copper a Reactions add sodium : ' blue orange sample burner would mash the flame flame + 20h Cu COM) > Cag) 2 ( s) + 20h - > Cag) agg " ( 111 Fe } + ( aq ) : + 301-1 Fe COH) } - caq) s ( s) - v brown precipitate ③ testing for ammonium ions ( Nhut ) Method add Result : : A - ☆ hydroxide sodium choking colourless gas is s which is ammonia > to test > damp litmus paper > - Fe ( OH )z ( s ) green precipitate Iron _ am - Fez Cag , . brick red : + 3 ¥ test pure cipitate blue Iron ( II ) crimson red hydroxide - this this tells us : produced hold the gas turns over damp litmus blue that ammonia barium gas is present ¥ yellow = sodium " : - -1 . = : CUZ Cag ) 2 green ¥ sample bunsen luminous - : paper = = lithium calcium ¥ blue ¥ lilac - = green = copper potassium