Colligative Properties of Solutions: Concentration Effects
advertisement

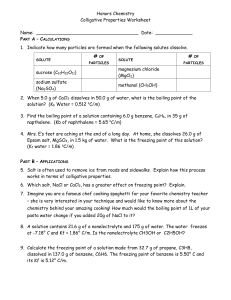
EFFECTS OF CONCENTRATION ON THE COLLIGATIVE PROPERTIES OF SOLUTIONS Objectives: 1. Describe the effect of concentration on the colligative properties of solutions (STEM_GC11PPIIId-f-115) Sub-tasks: 1. Define colligative and colligative properties. 2. Describe the effect of solute concentration on various solution properties: vapor pressure, boiling point, freezing point, and osmotic pressure. 3. Differentiate the colligative properties of nonelectrolyte solutions and of electrolyte solutions. (STEM_GC11PP-IIIdf-116 ), 4. Calculate boiling point elevation and freezing point depression from the concentration of a solute in a solution. (STEM_GC11PP-IIId-f-117) Colligative Properties Colligative came from a Latin word “colligatus” which means “depending on the collection” or it means, “grouped together. Changes in colligative properties depend only on the number of solute particles present, not on the identity of the solute particles. Among colligative properties are Vapor pressure lowering Boiling point elevation Melting point depression Osmotic pressure Let try the figure out this statement: ” It is a hot summer day and you have a picnic at the park or beach front with your classmates, friends or relatives with watermelon and “dirty ice cream”. Mmmmmm….. tastes good… refreshing…. The ice cream is an old-fashioned homemade kind ice cream. The kind of where the maker has a tub full of mix of ingredients immersed in a bigger tub filled with ice and salt. But wait a minute, why salt? Why the ice cream vendor does add salt to the ice? Colligative Properties of Nonelectrolyte Solutions Nonvolatile solutes lower the vapor pressure of a solvent by an amount proportional to the solute mole fraction. Vapor-Pressure Lowering P1 = X1 P 0 1 Raoult’s law P 10 = vapor pressure of pure solvent X1 = mole fraction of the solvent If the solution contains only one solute: X1 = 1 – X2 P 10 - P1 = DP = X2 P 10 X2 = mole fraction of the solute 13.6 Vapor-Pressure Lowering Calculate the vapor pressure of a solution made by dissolving 50.0 g glucose, C6H12O6, in 500 g of water. The vapor pressure of pure water is 47.1 torr at 37°C . Ans. 46.63 torr Boiling point is the temperature where the vapor pressure of the liquid equals the prevailing atmospheric pressure. Water boils at 100 0C when its vapor pressure equals the atmospheric pressure. When a nonvolatile solute, such as NaCl + H2O is added to a pure water or solvent, boiling point becomes higher than that of pure water. Boiling-Point Elevation When a nonvolatile solute, such as NaCl + H2O is added to a pure water or solvent, boiling point becomes higher than that of pure water. SOLVENT BOILING POINT (0C) 100 Kb (0C kg/mol) 0.51 Acetone 55.95 1.71 CCl4 76.8 5.02 Benzene 80.1 2.63 Chloroform 61.2 3.63 Water Boiling point of solution and Kb of common solvents. Boiling-Point Elevation DTb = Tb – T b0 T b0 is the boiling point of the pure solvent T b is the boiling point of the solution Tb > T b0 DTb > 0 DTb = Kb m m is the molality of the solution Kb is the molal boiling-point elevation constant (0C/m) 13.6 The colligative law states that, “the boiling point elevation is directly proportional to the molal concentration of the solution.” A solution of 10.0 g of a nonvolatile, nondissociating compound dissolved in 0.200 kg of benzene boils at 81.2 °C. Calculate the molecular weight of the compound. Ans. 119.5 g/mol Freezing-Point Depression The normal freezing point of a liquid is the temperature at which a liquid becomes a solid at 1 atm. When a solute is added to a pure solvent, the solute particle interrupts and reduces the attractive forces that will hold the solvent molecules together to form into a solid state. Temperature must be lowered to enable the solvent molecule to bind and come closer together. The presence of a nonvolatile solute lowers the freezing point of a pure solvent. Freezing-Point Depression DTf = T 0f – Tf T 0 Tf f is the freezing point of the pure solvent is the freezing point of the solution T 0f > Tf DTf > 0 DTf = Kf m m is the molality of the solution Kf is the molal freezing-point depression constant (0C/m) 13.6 SOLVENT FREEZING POINT (0C) 0 Kf (0C kg/mol) Benzene 5.5 5.12 Acetic Acid 16.6 3.90 CCl4 22.8 29.8 Camphor 179 39.7 Naphthalene 80.2 6.80 Water Figure 1. Freezing point of Solution Source: General Chemistry by: Tabajura, Jr. G.D. 1.86 How many grams of pyrazine (C4H4N2) would have to be dissolved in 1.50 kg of carbon tetrachloride to lower the freezing point by 4.4 °C? The freezing point constant for carbon tetrachloride is 30. °C/m. Ans. 17.6 g 13.6 Osmotic Pressure (p) Osmosis is the selective passage of solvent molecules through a porous membrane from a dilute solution to a more concentrated one. A semipermeable membrane allows the passage of solvent molecules but blocks the passage of solute molecules. Osmotic pressure (p) is the pressure required to stop osmosis. dilute more concentrated 13.6 Osmotic Pressure (p) High P Low P p = MRT M is the molarity of the solution R is the gas constant T is the temperature (in K) 13.6 A cell in an: isotonic solution hypotonic solution hypertonic solution 13.6 1. Hypertonic solution. If a cell is placed in a hypertonic solution, there will be a net flow of water out of the cell, and the cell will lose volume. A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. 2. Hypotonic solution. If the cell is placed in a hypotonic solution, there will be a net flow of water into the cell, and the cell will gain volume. If the solute concentration outside the cell is lower than inside the cell, and the solutes cannot cross the membrane, then that solution is hypotonic to the cell. 3. Isotonic solution. If the solute concentration outside the cell is the same as inside the cell, and the solutes cannot cross the membrane, then that solution is isotonic to the cell. The cell would be normal in shape. Sample Problem: 1. A very dilute solution, 0.0010 M sugar in water, is separated from pure water by an osmotic membrane. What osmotic pressure developed at 25oC? The gas constant R = 0.0821 L atm / mol K. 2. A solution is made by dissolving 13.0 g of sucrose, C12H22O11, in 117 g of water, producing a solution with a volume of 125 mL at 20.°C. What is the expected osmotic pressure at 20.°C? Colligative Properties of Nonelectrolyte Solutions Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. Vapor-Pressure Lowering P1 = X1 P 10 Boiling-Point Elevation DTb = Kb m Freezing-Point Depression DTf = Kf m Osmotic Pressure (p) p = MRT 13.6 Colligative Properties of Electrolyte Solutions 0.1 m Na+ ions & 0.1 m Cl- ions 0.1 m NaCl solution Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. 0.1 m NaCl solution van’t Hoff factor (i) = 0.2 m ions in solution actual number of particles in soln after dissociation number of formula units initially dissolved in soln i should be nonelectrolytes NaCl CaCl2 1 2 3 13.7 Chemists compare the degree of dissociation of electrolytes at different dilutions by a quantity called the van't Hoff factor, van’t Hoff factor (i) as the number of particles each solute formula unit breaks apart into when it dissolves Colligative Properties of Electrolyte Solutions Boiling-Point Elevation DTb = i Kb m Freezing-Point Depression DTf = i Kf m Osmotic Pressure (p) p = iMRT 13.7 Determine the freezing point of 1.77 m solution of NaCl in H2O. Solution For NaCl, we need to remember to include the van’t Hoff factor, which is 2. Otherwise, the calculation of the freezing point is straightforward: ΔTf = (2)(1.77 m)(1.86°C/m) = 6.58°C This represents the change in the freezing point, which is decreasing. So we have to subtract this change from the normal freezing point of water, 0.00°C: 0.00 − 6.58 = −6.58°C