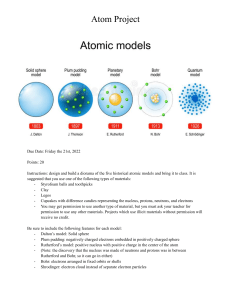
Lecture: 1 Modern Physics Relativity: Galileo observed that if a ship moved uniformly on the sea, a sailor could not distinguish between the situations either the ship is in motion and the sea is at rest or the ship is at rest and the sea is in motion. In Galileo’s view- the only motion that is measurable is the relative motion between the ship and the sea; hence the term “Relativity” was manifested first ever. To consider something relative to other is called relativity. There are two parts of Einstein’s theory, viz, Special relativity and general relativity. Special relativity (1905), treats problems that involve inertial frame of reference. General relativity (1915), treats problems that involve non inertial frame of reference. Frame of Reference: The co-ordinate system, relative to which any measurement can be done to specify the state of rest or motion of an object, is known as the frame of reference. When we have the origin and the directions in which to measure the distance from the origin, and a clock to measure the time, we say that we have a frame of reference or simply a frame. Inertial Frame: An inertial frame of reference is one in which Newton’s first law of motion holds good. In this frame, an object at rest remains at rest and an object in motion continues to move at constant velocity if no force acts on it. Any frame of reference that moves at constant velocity relative to an inertial frame is itself an inertial frame. Non-inertial Frame: A reference frame in which a body is accelerated without being acted upon by external force is called a non-inertial frame of reference. Newton’s laws are not valid in such a frame of reference. Failure of Newtonian Mechanics: For an electron accelerated through a 10 MeV potential difference, a value reasonable easy to obtain, the speed v equals 0.9988c. If the energy of 10 MeV is increased by a factor of four (to 40 MeV) experiment shows that the sped is not doubled to 1.9976 c as we might expect from the 1 Newtonian relation 𝐾𝐸 = 2 𝑚𝑣 2 but remain below c; it increases only from 0.9988 c to 0.9999 c, a chance of 0.11%. Why Galilean transformation introduced? Newton’s laws of motion does not tell us whether there is one or many inertial frames of reference, nor, if there is more than one, how we are to relate the coordinates of an event as observed from the one inertial reference frame to the coordinates of the same event as observed in other inertial reference frame. Galilean Transformation: The transformation of co-ordinates of a particle from one inertial frame of reference to another is called the Galilean transformation. Let us consider we are in an inertial frame of reference S and the coordinates of an event that occurs at the time t are (x, y, z). An observer located in a different inertial frame S which is moving with respect to S at constant velocity v, will find that the same event occurs at the time t and has the coordinates (x, y, z). Let us suppose that at t = t = 0 , S and S were coincide. For convenience, let v is in the +x direction as shown in the figure. Galilean transformation equations 𝑥 ′ = 𝑥 − 𝑢𝑡 𝑦′ = 𝑦 𝑧′ = 𝑧 𝑡′ = 𝑡 Inverse Galilean Transformation equations 𝑥 = 𝑥 ′ + 𝑢𝑡′ 𝑦 = 𝑦′ 𝑧 = 𝑧′ 𝑡 = 𝑡′ Postulates of special theory of relativity: 1. Principle of equivalence of physical laws: “The laws of physics are the same in all inertial frame of reference. No preferred inertial system exists”. 2. Principle of constancy of velocity of light: “The velocity of light in free space (vacuum) has the same value c 2.998×108 m/s in all inertial frames of reference, independent of the relative velocity of the source and observer”. Failure of Galilean transformation: The second postulate calls for the same value of the speed of light c when determined in S or S. This contradicts with Galilean transformation. If we measure the speed of light in the x-direction in the S system to be c, however, in the S system it will be 𝑐′ = 𝑐 − 𝑣 Clearly a different transformation is required if the postulates of special relativity are to be satisfied. Concept of Ether: Huygens' assumed that light wave propagated through the medium named as ‘Luminiferous Ether’. But problems arose when Ether is attributed to some strange properties: 1. Since light passes through vacuum, so Ether must be massless and of zero density and must have perfect transparency to account for its undetectability. 2. As the velocity of wave propagation depends on the elasticity of the medium, Ether must be highly elastic. Thus, the whole free space must be filled up with such an elastic medium to sustain the vibration of light waves. A solution of Maxwell’s equation gives the speed of light, 𝑐= 1 = 2.998x108 m/sec 𝜇𝑜 ε 𝑜 This is now well known that light waves are transverse electromagnetic waves and do not need any medium. Thank YOU Lecture: 2 Modern Physics Concept of Ether: Huygens' assumed that light wave propagated through the medium named as ‘Luminiferous Ether’. But problems arose when Ether is attributed to some strange properties: 1. Since light passes through vacuum, so Ether must be massless and of zero density and must have perfect transparency to account for its undetectability. 2. As the velocity of wave propagation depends on the elasticity of the medium, Ether must be highly elastic. Thus, the whole free space must be filled up with such an elastic medium to sustain the vibration of light waves. A solution of Maxwell’s equation gives the speed of light, 𝑐= 1 = 2.998x108 m/sec 𝜇𝑜 ε 𝑜 This is now well known that light waves are transverse electromagnetic waves and do not need any medium. Michelson-Morley Experiment: A beam of light (parallel wave) from a source S is split by a partially silvered mirror into two coherent beams. ➢ Beam (1) is transmitted to P and ➢ Beam (2) is reflected from P. ➢ Beam (1) is reflected back to P by the mirror B and ➢ Beam (2) is reflected back to P by the mirror A. The interference will be constructive or The returning Beam (1) is reflected by P and Beam (2) is transmitted through P and they enter into the telescope T, where they interfere. destructive c = speed of light depending on the path difference of the two beams. Earth Velocity The mirror P is inclined at 45 to the incident beam directions. Earth moves with a velocity v along SP. Here, D = PB = BP. Time taken by Beam (1) travel from P to B and back to P is Binomial Expansion 1 + 𝑥 𝑛 = 1 + 𝑛𝑥 if x < < 1 B The path of Beam (2) travelling from P to movable mirror A and back is a cross-stream path through the ether to return to the mirror p. Transit time tA is given by tA = t1 + t2 = t1 + t1 = 2 t1 Now, D2 + V2t12 = c2t12 Or, t12 (c2 – v2) = D2 Or, t12 = D2 / (c2 – v2) 𝑡12 = Or, 𝐷2 𝑣2 𝑐 2 1− 2 𝑐 𝐷 (1 − 𝑐 Or, 𝑡1 = Therefore, tA = 2t1 = 2𝐷 𝐶 𝑣 2 −1 𝐷 )2= 𝐶 𝑐2 1+ 𝑣2 2𝑐 2 1+ 𝑣2 2𝑐 2 The difference in time taken by the two beams of light for a round trip is If we rotate the apparatus by 90, the time difference will be reversed as A→B In that case, Therefore, the change in these two time difference is Then the corresponding path difference is For constructive interference we know that, 2 Dv 2 path difference, d = n = 2 c So the fringe shift, In actual experiment, Michelson and Morley used D = 10 m, = 5000Å and the earth speed v = 3×104 m/sec. Then fringe shifts 2 10m (3 104 m / s) 2 n= = 0.4 fringe −7 8 2 (5 10 m) (3 10 ) ➢ The arrangement was capable of measuring 1/100th of a fringe shift. But, actually no fringe shift was found during this measurement. ➢ The experiment was performed at different seasons and different places with the same result every time and no fringe shift was detected. ➢ Thus, it was concluded that the relative velocity between the earth and the Ether is zero and there is no fringe shift at all. The result of Michelson-Morley experiment was explained by Einstein and he concluded that: 1. There is no ether frame in space, and 2. The speed of light in free space is invariant i.e. it is constant and is independent of the motion of source, medium or the observer. Derivation of Lorentz Transformation Equations: For the constancy of velocity of light, we have to introduce the new transformation equations which fulfill the following requirements:1. The speed of c must have the same value in every inertial frame of reference. 2. The transformations must be linear and for low speeds u<<c, they should approach the Galilean transformations. 3. They should not be based on “absolute time” and “absolute space”. A reasonable guess about the nature of the correct relationship between x and x is x = k ( x − ut ) - - - - - - - - - (1) Here k is a factor that does not depend on either x or t but may be a function of u. The corresponding equation for x in terms of x and t x = k ( x + ut ) - - - - - - - - - (2) The factor k must be the same in both frames of reference since there is no difference between S and S other than in the sign of u. Let us consider that a light pulse that starts at the origin of S at t = 0. Since we have assumed that the origins are coincident at t = t = 0, the pulse also starts at the origin of S at t = 0. Einstein’s postulates require that the equation for the x-component of the wave front of the light pulse is x = ct in frame S and x = ct in frame S. Substituting ct for x and ct for x in Eq. (1) and (2), we get ct = k (ct + ut ) = k (c + u)t - - - - (3) and ct = k (ct − ut ) = k (c − u)t From equation (3), - - - - (4) t c = t k (c + u ) Similarly, from equation (4), t k (c − u ) = t c and thus, we get 2 c k (c − u ) c = k2 = 2 k (c + u ) c c − u2 1 1 k = k = 2 2 1− u / c 1− u2 / c2 2 Substituting x = k ( x − ut ) for x in x = k ( x ' + ut ' ) , x = k[k ( x − ut ) + ut ] = k 2 ( x − ut ) + kut or, x − k 2 ( x − ut ) t = ku x− = = = ( x − ut ) (1 − u 2 / c 2 ) 1− u 2 / c2 u x − u 2 / c 2 x − x + ut u 1− u2 / c2 ut − u 2 / c 2 x u 1− u2 / c2 - - - - - - - - - (5) we get u x 2 c = 1− u2 / c2 t− u t− 2 x u c t = = k (t − 2 x) c 1− u2 / c2 The Lorentz transformation set is x = k ( x − ut ) y = y z = z ux t = k (t − 2 ) c The Inverse Lorentz transformation set is x = k ( x + ut ) y = y z = z ux t = k (t + 2 ) c - - - - - - - - - (6) Thank YOU Lecture: 3 Modern Physics Derivation of Lorentz Transformation Equations: For the constancy of velocity of light we have to introduce the new transformation equations which fulfill the following requirements:1. The speed of c must have the same value in every inertial frame of reference. 2. The transformations must be linear and for low speeds u<<c, they should approach the Galilean transformations. 3. They should not be based on “absolute time” and “absolute space”. A reasonable guess about the nature of the correct relationship between x and x is x = k ( x − ut ) - - - - - - - - - (1) Here k is a factor that does not depend on either x or t but may be a function of u. Because the equations must have the same form in both S and S, we need only change the sign of u (in order to take into account the difference in the direction of relative motion) to write the corresponding equation for x in terms of x and t: x = k ( x + ut ) - - - - - - - - - (2) The factor k must be the same in both frames of reference since there is no difference between S and S other than in the sign of u. Let us consider that a light pulse that starts at the origin of S at t = 0. Since we have assumed that the origins are coincident at t = t = 0, the pulse also starts at the origin of S at t = 0. Einstein’s postulates require that the equation for the x-component of the wave front of the light pulse is x = ct in frame S and x = ct in frame S. Substituting ct for x and ct for x in Eq. (1) and (2), we get ct = k (ct + ut ) = k (c + u)t - - - - (3) and ct = k (ct − ut ) = k (c − u)t From equation (3), - - - - (4) t c = t k (c + u ) Similarly from equation (4), t k (c − u ) = t c and thus we get 2 c k (c − u ) c = k2 = 2 k (c + u ) c c − u2 1 1 k = k = 2 2 1− u / c 1− u2 / c2 2 Substituting x = k ( x − ut ) for x in x = k ( x ' + ut ' ) , x = k[k ( x − ut ) + ut ] = k 2 ( x − ut ) + kut or, x − k 2 ( x − ut ) t = ku x− = = = ( x − ut ) (1 − u 2 / c 2 ) 1− u 2 / c2 u x − u 2 / c 2 x − x + ut u 1− u2 / c2 ut − u 2 / c 2 x u 1− u2 / c2 - - - - - - - - - (5) we get u x 2 c = 1− u2 / c2 t− u t− 2 x u c t = = k (t − 2 x) c 1− u2 / c2 The Lorentz transformation set is x = k ( x − ut ) y = y z = z ux t = k (t − 2 ) c The Inverse Lorentz transformation set is x = k ( x + ut ) y = y z = z ux t = k (t + 2 ) c - - - - - - - - - (6) You have to read yourself: Length Contraction Time Dilation Einstien’s Mass And Energy Relation The relativistic addition of velocities Let a train move with a velocity v with respect to the ground. If a passenger on the train, move with a velocity u’ with respect to the train, then according to classical physics, the passenger's velocity relative to the ground u is just the vector sum of the two velocities, i.e. u = u‘ + v - - - - - - (1) Let S be the ground frame and S' the frame of the train, whose speed relative to the ground is v. The passenger's speed in the S' frame is u' and his position on the train as time goes on can be described by x’= u’t ‘ - - - - - - (2) Using Lorentz transformation equations we have, 𝑥′ = and 𝑡′ = 𝑥 − 𝑣𝑡 1− 𝑣 2 /𝑐 2 𝑣 𝑥 𝑐2 1 − 𝑣 2 /𝑐 2 𝑡− = 𝑢′ 𝑡 ′ - - - - - - - - - (3) - - - - - - - - - (4) [Using equ. (2)] From equation (3), 𝑥 − 𝑣𝑡 = 𝑢′ 𝑡 ′ 1 − 𝑣 2 /𝑐 2 𝑣𝑥 𝑐2 𝑢′ 𝑣𝑥 ′ =𝑢 𝑡− 2 𝑐 = 𝑢′ 𝑡 − 𝑜𝑟, 𝑢′ 𝑣𝑥 𝑥 + 2 = 𝑢′ + 𝑣 𝑡 𝑐 (𝑢′ + 𝑣) 𝑜𝑟, 𝑥= ×𝑡 𝑢′ 𝑣 1+ 2 𝑐 [ Using equation (4) ] - - - - - - - - - (5) If the passenger's speed relative to the ground be u, then his ground location as time goes on is given by x = ut - - - - - - - - - (6) Comparing eqn. (5) with eqn. (6), we obtain (𝑢′ + 𝑣) 𝑢= 𝑢′ 𝑣 1+ 2 𝑐 This is the relativistic addition of velocities. Case-1: If u' and v are very small compared to c, then the second term in the denominator of is negligible compared to one (1). Then above equation reduces to the classical result, i.e., u = u + v. Case-2: If, on the other hand, u' = c i.e., our passenger on the train is a light pulse, then (𝑐 + 𝑣) (𝑐 + 𝑣) (𝑐 + 𝑣) 𝑢= 𝑐𝑣 = 𝑣 = (𝑐 + 𝑣) = 𝑐 1+ 2 1+𝑐 𝑐 𝑐 Example: Two electrons leave a radioactive sample in opposite directions, each having a speed of 0.67c with respect to the sample. What is the speed of one electron relative to the other? Solution: Here v x = 0.67 c and u = 0.67c The speed of one electron relative to other v x + u 0.67c + 0.67c 1.34c vx = = = = 0.92c u 0.67c 1.45 1 + 2 v x 1 + 2 0.67c c c This is means that speed of one electron relative to the other is less than c. Relativity of mass Consider two frames of reference S and S'. S' is moving with a constant velocity v relative to S, in the positive x-direction. S' x ' Two exactly similar elastic balls A and B in the frame S' Same mass, moving in opposite direction with equal velocity u and –u. Alter collision they coalesce into one. According to the law of conservation of momentum mu + m (-u) = momentum of coalesced mass = 0 Thus, the coalesced mass must be at rest in S' frame. Consider the collisions with respect to the frame S. Let u1 and u2 be the velocities of the balls as observed from S. Then, and v is the relative velocity of coalesced mass, u1 is the velocity of m1 (A) and u2 is the velocity of m2 (B) with respect to frame S. Since the total momentum of the balls must be conserved, we have m1u1 + m2u2 = (m1 + m2)v From equations (i), (ii) and (iii), we get, (iii) Thank YOU Online Lecture – 4 Modern Physics Relativity of Simultaneity: Two events are said to be simultaneous if they occur at the same time. According to the relativity of simultaneity, if two observers are in relative motion, they will not agree as to whether two events are simultaneous. If one observer finds them to be simultaneous, the other generally will not. The simultaneity of the two events is not an absolute concept and depends on the frame of reference. In fact, each observer is correct in his own frame of reference. Two events that appear simultaneous to an observer A will not be simultaneous to an observer B if B is moving with respect to A. Let us consider an example to clarify the above statement: Figure: Two tennis ball ejected at a same time with same velocity in a moving train as observed (a) by an observer on the train itself and (b) by an observer standing on the ground. Let us consider two frames of reference S and S. The frame reference S is moving with velocity u relative to the frame of reference S along +ve direction of x-axis and the two events occur simultaneously in S. Since the events are simultaneous in frame S, therefore we have t1 = t2. If t1 and t 2 are the corresponding times of the same two events with respect to system S, then we have from Lorentz transformation equations:ux2 ux1 t = k ( t − ) and t1 = k (t1 − 2 ) 2 2 2 c c ux 2 ux1 ) − k ( t − ) 1 2 2 c c u ( x1 − x 2 ) =k since t1 = t 2 2 c t 2 − t1 = k (t 2 − Thus, if the events are simultaneous in frame S, t1 must be equal to t 2 or t 2 − t1 must be equal to zero, but it is not so because x1 is not equal to x2. Therefore, the same two events are not simultaneous in frame S. Relativistic kinetic energy (KE) to Classical KE We know and m0 c 2 E = mc 2 = 1− v2 / c2 1 K = m c − m0 c = − m 0 c = m0 c − 1 2 2 2 2 1− v / c 1− v / c 2 2 m0 c 2 2 2 Using the binomial approximation (1 + x) n 1 + nx with x 1 , For low speeds, v / c 1 1 K = m0 c 2 − 1 2 2 1− v / c 1 v2 2 = m0 c 1 + − 1 2 2 c 1 = m0 v 2 2 we get At low speed, the relativistic expression for the K.E. of a moving body reduces to the (9) classical one. Mass-less particles: We know, and relativistic momentum total energy p= m00vu E= m0 c 2 1− v / c 2 2 , 1− v2 / c2 (Case-1) When m0 = 0 and v < c, i.e., E = p = 0 i.e., A mass less particle with a speed less than that of light can have neither energy nor momentum. (Case-2) When mo = 0 and v = c, E = 0/0 and p = 0/0 which are indeterminate: E and p can have any values. The above equations are consistent with the existence of mass-less particles that possess energy and momentum provided that they travel with the speed of light. we can write Subtracting p 2c 2 2 2 2 m0 c 4 2 E = 1− v2 / c2 and from E2 , we get, m0 v 2 p = , 1− v2 / c2 2 or, m0 v 2 c 2 p c = 1− v2 / c2 2 2 2 2 m0 c 4 m0 v 2 c 2 2 2 2 E −p c = − 2 2 1− v / c 1− v2 / c2 c2 v2 2 2 = m0 c 2 − 2 2 2 c − v c − v 2 c 2 2 c − v 4 = m0 c 2 2 c −v 2 = m0 c 4 2 (12) E 2 = m0 c 4 + p 2 c 2 2 Therefore, for all particles, we have E = mo c 4 + p 2 c 2 = 2 Eo + p 2 c 2 2 According to this formula, if a particle exists m0 = 0, then the relationship between its energy and momentum is given by mass - less particles 𝑜𝑟, E = pc 𝑝= 𝐸 ℎυ = 𝑐 𝑐 Theory of Light There are different theories on the nature of the light. The important theories are as follows: 1. Newton's corpuscular particle theory: Isaac Newton stated in his Hypothesis of Light on 1672 that light was composed of corpuscles (tiny, light and elastic particles) which were emitted in all directions from a source. 2. Huygens, Wave Theory: Christiaan Huygens worked out a mathematical wave theory of light in 1679 and published it in his treatise on light in 1690. He proposed that light was emitted in all directions as a series of waves in a medium called the Luminiferous ether. 3. Maxwell's electromagnetic theory: Maxwell concluded that light was a form of electromagnetic radiation, he first stated this result in 1862. 4. Planck's Quantum theory: In 1900 Max Planck, attempting to explain black-body radiation, suggested that light energy is a combination of "quanta“. 5. Dual nature of light: It is established that light has both the nature of wave and particles. Planck's Quantum theory: Postulates of Planck's quantum theory: 1) Energy is not emitting continuously but discontinuously in the form of small packets of energy known as quanta of energy (photon). 2) The energy of each quantum is directly proportional to the frequency of radiation i.e., E directly proportional to ʋ, where ʋ is frequency? Photo-electric effect: W Smith around 1885 found that resistance of a metallic wire varies when sun ray is incident on it. W Smith was a telegraph operator. When electromagnetic radiation is incident on a metal surface then electrons are emitted from that surface, this phenomenon is known as Photoelectric effect. The emitted electrons are known as photoelectric electron and the current flow due to the emission of these electrons is known as photoelectric current. Characteristics (laws) of photoelectric emission: 1. Emission commences at the instant the surface starts to be irradiated. 2. For any incident radiation of a constant frequency ʋ, and if the accelerating potential V is constant, then the number of electron emitted per second, i. e., photoelectric current is proportional to the intensity of the radiation. 3. Emission occurs only if the frequency of the incident radiation is above some minimum frequency called the threshold frequency. The value of threshold frequency is different for different metal. With the increase in frequency of the incident radiation the energy of the emitted electron increases. Characteristics (laws) of photoelectric emission: 4. If both the intensity and frequency of the incident radiation are kept constant, as the accelerating potential is reduced from zero to negative values the photoelectric current does not immediately drops to zero. This proves that electrons are emitted with some definite velocity with sufficient enough to give kinetic energy to the electron so as to overcome the retarding electric field between the electrodes. When the negative potential is made at large enough as Vs then the current is reduced to zero. Vs is known as the stopping potential. Vs is independent of the intensity of the incident radiation. 5. Stopping potential varies linearly with the frequency. Thank YOU Lecture: 5 Modern Physics Failure of wave theory of light to explain Photoelectric effect: (i) According to wave theory Energy ∝ (Intensity)2 or, E ∝ I2 ➢ Light of low frequency with sufficient intensity is much effective than light of higher frequency with low intensity. But it is contradictory to the experimental result. (ii) It is equally difficult to explain that there is no time lag between the arrival of the radiation on the metal surface and the emission of photoelectrons. • Consider violet light incident on a sodium surface. • Detectable photoelectric current will result when 10-6 watt of electromagnetic energy is absorbed by 1 m2 of the surface. 1m2 1m 1m 1m Fig. 1: Sodium cube Now there are about 1019 atoms in a layer of sodium one atom thick and 1m2 in area. Consider light absorbed by 10 uppermost layers of sodium atom. Therefore, 10-6 watt is distributed by 1020 atoms. Therefore, energy received per atom is 10-26 watt = 10-26 J s-1 = 6.25 x 10-8 eV s-1 So it should be taken 1.6 x 107 sec to receive 1eV per atom. = 185 days If 2 eVis the work function of that metal, then almost 1 Year is required to emit electrons from that surface. But, practically, it is an instantaneous event. Einstein Photoelectric equation: Energy of incident Photon = Energy required for getting the electron free from the atom of metal surface + kinetic energy of the electron or, hʋ = hʋo + ½ mv2 Determination of Planck's Constant: The stopping potential for a particular metal were determined for different frequencies. A graph is plotted with ʋ along x-axis and Vs along y-axis. The resulting graph is a straight line. Light-matter interaction: The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. Thomson scattering is the elastic scattering of electromagnetic radiation by a free charged particle. It is the low-energy limit of Compton scattering; the particle's kinetic energy and photon frequency do not change as a result of the scattering. This limit is valid as long as the photon energy is much smaller than the mass-energy of the particle/electron: hʋ << mc2, i. e., the wavelength of the light is much greater than the Compton wavelength of the particle. Compton scattering, discovered by Arthur Holly Compton, is the scattering of a photon by a charged particle, usually an electron. It results in a decrease in energy (increase in wavelength) of the photon. Part of the energy of the photon is transferred to the recoiling electron. Pair production is the creation of a subatomic particle and its antiparticle from a photon. Examples include creating an electron and a positron, a muon and an antimuon, or a proton and an antiproton etc. Pair production often refers specifically to a photon creating an electron–positron pair near a nucleus. For pair production to occur, the incoming energy of the photon must be above a threshold of at least the total rest mass energy of the two particles, and the situation must conserve both energy and momentum. However, all other quantum numbers (angular momentum, electric charge, lepton number) are also conserved. Thus the created particles shall have opposite values of each other. For instance, if one particle has electric charge of +1 the other must have electric charge of −1. The probability of pair production in photon–matter interactions increases with photon energy and also increases approximately as the square of atomic number of the nearby atom. The energy of a photon can be converted into an electron–positron pair: The photon's energy is converted to particle mass in accordance with Einstein’s equation, E = m ⋅ c2 Photodisintegration is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle (such as, the neutron and proton, etc.). The incoming gamma ray effectively knocks one or more neutrons, protons, or an alpha particle out of the nucleus. The reactions are called (γ,n), (γ,p), and (γ,α). Photofission is a process in which a nucleus, after absorbing a gamma ray, undergoes nuclear fission and splits into two or more fragments. Applications of photo-electric effect Photo-electric cell: Two types (1) Vacuum tube (2) Gas filled tube C => Semi-cylindrical plate coated with potassium or caesium known as emitter. A => A wire of platinum or nickel fixed along the axis of the cylinder known as collector. When photons are incident on the emitter then photoelectric current is produced in the circuit is detected by the galvanometer. If the tube is vacuum then current is very weak. On the other hand, if the tube is filled by gas (usually Argon or Helium) then photo-electron bombarded the gas molecules. In this way gas molecules are ionized. As a result a large current is flow through the circuit. Applications of the photoelectric cell The photoelectric cell is used to automatic turn on and off the street bulbs. The photoelectric cell is used to automatic traffic control. The photoelectric cell is used in exposure meter. The exposure meter is used along with a camera to know the correct time of exposure for having a good photograph. The photoelectric cell is used in lux-meter. It is used to determine the intensity of light. The photoelectric cell is used in a burglar alarm. This device is kept near to door to protect form a thief. Photomultiplier tube: A photomultiplier tube, useful for light detection of very weak signals, is a photo-emissive device in which the absorption of a photon results in the emission of an electron. Photomultipliers are typically constructed with an evacuated glass tube, containing a photocathode, several dynodes, and an anode. Incident photons strike the photocathode material. Electrons are ejected from the surface are directed by the focusing electrode toward the electron multiplier, where electrons are multiplied by the process of secondary emission. The electron multiplier consists of a number of electrodes called dynodes. Each dynode is held at a more positive potential, by ≈100 Volts, than the preceding one. A primary electron leaves the photocathode with the energy of the incoming photon. A small group of primary electrons is created by the arrival of a group of initial photons. The primary electrons move toward the first dynode because they are accelerated by the positive electric field. They each arrive with ≈100 eV kinetic energy. Upon striking the first dynode, more low energy electrons are emitted, and these electrons are in turn accelerated toward the second dynode. The geometry of the dynode chain is such that a cascade occurs with an exponentiallyincreasing number of electrons being produced at each stage. For example, if at each stage an average of 6 new electrons are produced for each incoming electron, and if there are 11 dynode stages, then at the last stage one expects for each primary electron about 611 ≈ 3x108 electrons. This last stage is called the anode. This large number of electrons reaching the anode results in a sharp current pulse that is easily detectable. Photo-voltaic cell: In the case of photo-electric cell an external potential is required. But the photo-voltaic cell is a self-generating cell where the electrons ejected by the light, themselves produce a potential difference between two plates, which causes the current to flow through the external circuit, even though no external battery is applied. The most commonly used photovoltaic cell are: iron-selenium cells In this, selenium layer is placed on an iron base. An extremely thin semi-transparent layer of silver is formed on the selenium to act as a front electrode. A contact ring on the silver layer acts as one electrode and the iron base acts as the other electrode. Solar cell construction and working principle: Solar cell is a device that convert the solar energy into the electrical energy. The basic principle behind the function of solar cell is based on photo-voltaic cell. The electricity supplied by the solar cell is DC. Which is same like provided by a battery but a little bit different in the sense the battery is providing constant voltage. Construction of solar cell Mainly solar cell is constructed using the crystalline silicon that consists of a ntype semiconductor. This is the upper layer also known as emitter layer. The second layer is p-type semiconductor layer known as base layer. Both the layer are sandwiched and hence there is formation of p-n junction between them. The surface is coated with anti-reflecting coating to avoid the loss of incident light energy due to reflection. Working of solar cell As soon as the solar cell is exposed to sunlight, the solar energy is absorbed by semiconductor materials. Due to this absorbed energy the phenomena of photo-voltaic occurs and electrons are liberated and produce the external DC current. The DC current is converted into 220 volt AC current using and inverter for different applications. An atom of silicon has 14 electrons, the outer shell has four electrons. Therefore a silicon atom will always look for ways to feel up its last shell and to do this it will share electron with four nearby atoms. Now we use phosphorus (with 5 electrons in its outer shell). Therefore when it combines with silicon, one electron remains free. When energy is added to pure silicon it can cause few electron to break free of their bonds and leave their atoms. These are called free carriers, which move randomly around the crystalline lattice looking for holes to fall into and carrying an electrical current. The other part of solar cell is doped with the element boron (with 3 electrons in its outer shell) to become p-type silicon. Now when these two type of silicone interact, an electric field form at the junction which prevents more electrons to move to p-site. When photon hits solar cell, its energy break apart electron-hole pairs. Each photon with enough energy will normally free exactly one electron, resulting in a free hole as well. If this happens close enough to the electric field, this causes disruption of electrical neutrality, and if we provide an external current path, electron will flow. This electron flow provides the current. Electron-hole formation Types of solar cell and efficiency levels Solar panel/solar array and Solar module You have to read yourself Automatic bell alarm circuit? Math problems? Compton Effect: When a photon of energy hʋ strikes a free electron, then energy is communicate between them. In this case the incident photon loss its energy. Consequently it acquire a lower frequency or higher wavelength than that of incident photon. These change in wavelength or frequency is known as Compton effect. Generally, the electron considered to be at rest and the photon is usually considered to be an energetic photon such as an X-ray photon. Compton Theory: According to the law of conservation of energy, ℎ𝜗 + 𝑚𝑜 𝑐 2 = h𝜗 ′ + 𝑚𝑐 2 -------- (1) According to the law of conservation of momentum, Along the direction of incident photon – (x-axis), ℎ𝜗 ℎ𝜗 ′ -------- (2) +0= 𝑐𝑜𝑠𝜃 + 𝑚𝑣 𝑐𝑜𝑠∅ 𝑐 𝑐 Along the direction perpendicular to the incident photon – (y-axis), 0+0= ℎ𝜗′ 𝑐 sin𝜃 − 𝑚𝑣 𝑠𝑖𝑛∅ -------- (3) From equations (2) and (3) 𝑚𝑣𝑐 𝑐𝑜𝑠∅ = ℎ𝜗 − ℎ𝜗 ′ 𝑐𝑜𝑠𝜃 -------- (4) 𝑚𝑣𝑐 𝑠𝑖𝑛∅ = ℎ𝜗 ′ 𝑠𝑖𝑛𝜃 -------- (5) 4 2 + 5 2 𝑚2 𝑣 2 𝑐 2 = ℎ2 𝜗 2 + ℎ2 𝜗 ′2 𝑐𝑜𝑠 2 𝜃 − 2ℎ2 𝜗𝜗 ′ 𝑐𝑜𝑠𝜃 + ℎ2 𝜗 ′2 𝑠𝑖𝑛2 𝜃 or, 𝑚2 𝑣 2 𝑐 2 = ℎ2 𝜗 2 + ℎ2 𝜗 ′2 − 2ℎ2 𝜗𝜗 ′ 𝑐𝑜𝑠𝜃 -------- (6) From equ. (1) 𝑚𝑐 2 = ℎ𝜗 − ℎ𝜗 ′ + 𝑚0 𝑐 2 or, 𝑚2 𝑐 4 = ℎ2 𝜗 2 + ℎ2 𝜗 ′2 + 𝑚02 𝑐 4 − 2ℎ2 𝜗𝜗 ′ − 2ℎ𝜗 ′ 𝑚0 𝑐 2 + 2ℎ𝜗𝑚0 𝑐 2 -------- (7) (7)-(6) 𝑚2 𝑐 2 𝑐 2 − 𝑣 2 = 𝑚02 𝑐 4 − 2ℎ2 𝜗𝜗 ′ − 2ℎ𝜗 ′ 𝑚0 𝑐 2 + 2ℎ𝜗𝑚0 𝑐 2 + 2ℎ2 𝜗𝜗 ′ 𝑐𝑜𝑠𝜃 We know, 𝑚= 𝑚0 𝑣2 1− 2 𝑐 2 2 𝑚 𝑐 0 𝑚2 = 2 𝑐 − 𝑣2 𝑚2 𝑐 2 − 𝑣 2 = 𝑚02 𝑐 2 𝑚2 𝑐 2 𝑐 2 − 𝑣 2 = 𝑚02 𝑐 4 ---- (8) From equ. (8) 𝑚02 𝑐 4 = 𝑚02 𝑐 4 − 2ℎ2 𝜗𝜗 ′ 1 − 𝑐𝑜𝑠𝜃 + 2ℎ𝑚0 𝑐 2 (𝜗 − 𝜗 ′ ) or, 2ℎ𝑚0 𝑐 2 𝜗 − 𝜗 ′ = 2ℎ2 𝜗𝜗 ′ (1 − 𝑐𝑜𝑠𝜃) or, 𝜗 − 𝜗′ ℎ = (1 − 𝑐𝑜𝑠𝜃) 𝜗𝜗 ′ 𝑚0 𝑐 2 or, 1 1 ℎ − = (1 − 𝑐𝑜𝑠𝜃) 𝜗′ 𝜗 𝑚0 𝑐 2 or, 𝑐 𝑐 ℎ − = (1 − 𝑐𝑜𝑠𝜃) 𝜗′ 𝜗 𝑚0 𝑐 or, λ′ or, ℎ −λ= (1 − 𝑐𝑜𝑠𝜃) 𝑚0 𝑐 Δλ = ℎ (1 − 𝑐𝑜𝑠𝜃) 𝑚0 𝑐 Δλ = 2ℎ 𝑚0 𝑐 𝑠𝑖𝑛2 𝜃 2 Thank YOU Lecture: 6 Modern Physics Derive an expression for (a) Kinetic energy of recoil electron and (b) Direction of recoil electron Math problem (Compton effect) Wave Particle duality/de-Broglie hypothesis: In 1924, de-Broglie suggested that matter, like radiation, has dual nature, i., e., matter which is made up of discrete particles (atoms, protons, electrons etc) might exhibit wave like properties. de-Broglie wavelength: Let the quantity that undergoes periodic changes giving rise to matter waves be Ψ. Let its value at a point xo, yo, zo at any instant of time to be given by, Ψ = Ψ𝑜 sin 2π𝜗𝑜 𝑡𝑜 -------- (1) Where Ψo is the amplitude and 𝜗o be the frequency as observed at rest with respect to the particle. By applying the Lorentz transformation equation, we can write, 𝑣𝑥 𝑐2 𝑣2 1− 2 𝑐 𝑡− 𝑡𝑜 = -------- (2) From equations (1) and (2) we get, 𝑣𝑥 ) 𝑐2 𝑣2 1− 2 𝑐 (𝑡 − Ψ = Ψ𝑜 sin 2π𝜗𝑜 -------- (3) This equation may be compared with, 𝑥 𝑦 = 𝐴 sin 2π 𝜗(𝑡 − ) 𝑢 -------- (4) By comparing equations (3) and (4) we get, 𝑐2 𝑢= 𝑣 and 𝜗= 𝜗𝑜 𝑣2 1− 2 𝑐 -------- (5) From Einstein’s mass-energy relation, ℎ𝜗𝑜 = 𝑚𝑜 𝑐2 => 𝑚𝑜 𝑐 2 𝜗𝑜 = ℎ -------- (6) From equations (5) and (6) we get, 𝑚𝑜 𝑐 2 𝑚𝑐 2 𝜗= = 2 ℎ 𝑣 ℎ 1− 2 𝑐 Therefore, wavelength of matter wave, λ= 𝑉𝑒𝑙𝑜𝑐𝑖𝑡𝑦 𝑢 = 𝐹𝑟𝑒𝑞𝑢𝑒𝑛𝑐𝑦 𝜗 Using equations (5) and (7) we get, 𝑐2 𝑐 2ℎ ℎ 𝑣 λ= = = 𝑚𝑐 2 𝑣𝑚𝑐 2 𝑚𝑣 ℎ λ= ℎ 𝑚𝑣 -------- (7) Maths: de-Broglie wavelength Rutherford's atom model: The main points of Rutherford's atom model are# An atom consists of a positively charged nucleus, which is surrounded by electrons moving around it as planet revolve around the sun . # Electrons and the nucleus are held together by coulombic force of attraction. Gold atom Drawbacks/Limitations of Rutherford’s Atom Model: Rutherford’s model explains the structure of atom in very simple way, but it suffers from the following drawbacks: # Rutherford's atom model could not explain the stability of the atom. According to Rutherford's model electrons revolve around the nucleus as planets revolve around the sun according to classical theory of mechanics, during uniform revolution, any body accelerates. An accelerating charged particle must emits radiation and lose energy. As a result, the electron will follow a spiral path, and ultimately fall into the nucleus . In such cases the atom would be highly unstable and would collapse. However, this is not consistent with real-world observations as the atoms are stable. # The Rutherford Model of atom does not say anything about the arrangement of electrons in an atom. Bohr’s Atom Model: In 1913 Bohr proposed an atom model to resolve the problems of the Rutherford's atom model. Bohr proposed that classical mechanics break down into the atom model. Bohr applied quantum mechanics in Rutherford's atom model. Bohr’s proposal are: 1. The angular momentum of the electrons are whole number ℎ ℎ multiple of , 𝑖. 𝑒. , 𝑛 . Thus, the angular momentum is quantized. 2𝜋 2𝜋 This means that electrons can have only certain radii called energy levels. 2. The electrons of an atom are orbiting along some particular orbits. During rotation electrons never emit any radiation (energy). 3. An electron can jump from one orbit of energy E1 to another orbit of energy E2. In doing so electron either emits or absorbs an electromagnetic radiation of frequency ϑ, so that E1 E2 = hϑ. • The Bohr model explain why the spectra are not continuous because the electron are in only certain discrete energy level or state. 3 ℎ 2𝜋 2 ℎ 2𝜋 ℎ 2𝜋 An electron revolves around the nucleus, in the n th orbit of radius rn then, Limitation of Bohr’s atom model: Bohr was successful in introducing the idea of quantum energy state, but it has some limitations: 1. Bohr’s model could not explain the spectra of atoms containing more than one electron. His theory can only explain the hydrogen spectrum or ions containing one electron, eg., He+, Li2+. 2. In the presence of a magnetic field, each spectral line get split up into closely spaced line (Zeeman effect) could not be explained by Bohr’s model. Similarly, the splitting of spectral line under the effect of applied electric field (Stark effect), could not be explained by Bohr’s model. de-Broglie hypothesis on atom Model: de-Broglie suggested that, 1. The motion of electron can be represented by standing matter wave. 2. Only certain wavelength can exist, i. e., its energy can be only certain discrete values (the motion of electron is quantized). According to de-Broglie, 2𝜋𝑟𝑛 = 𝑛λ ℎ We know matter wavelength, λ = where, n = 1, 2, 3, - - - 𝑚𝑣 ℎ 𝑚𝑣 ℎ 𝑛 2𝜋 or, 2𝜋𝑟𝑛 = 𝑛 or, m𝑣𝑟𝑛 = 𝐴𝑛𝑔𝑢𝑙𝑎𝑟 𝑀𝑜𝑚𝑒𝑛𝑡𝑢𝑚 = 𝑛 ℎ 2𝜋 Which satisfy the Bohr postulates. Fig.1: Standing wave fit to a circular Bohr orbit. In this particular diagram three wavelength are fit to the orbit. Corresponding to the n = 3 energy state of the Bohr theory. Pauli's exclusion principle In 1925, Wolfgang Pauli The Pauli exclusion principle says that no two identical fermions can simultaneously occupy the same quantum state. All fermions, such as protons, neutrons, Quarks (up and down), leptons (electrons, electron neutrinos, muons, muon neutrinos, taus, and tau neutrinos), etc., obey Fermi-Dirac statistics; this includes obeying the Pauli’s exclusion principle. their spin is always some odd whole-number multiple of one-half. The Pauli exclusion principle does not apply to bosons: these are particles that obey Bose-Einstein statistics; they all have integer values of spin. Photons, gluons, gravitons, and the W, Z and Higgs bosons, etc., are all bosons. Quantum numbers of electron should be considered as address of each electron within an atom, each address has four components. Pauli’s Exclusion principle states that no two electrons in an atom can have the same four quantum numbers. If two electrons occupy the same orbital, they must have different spins. Pauli’s Exclusion Principle limits the numbers of electrons that a shell or a subshell may contain. The four quantum numbers Principal Quantum Number n This quantum number describes the electron shell or energy level of an atom. The value of the principal quantum number can be any integer with a positive value that is equal to or greater than one. A larger value of the principal quantum number implies a greater distance between the electron and the nucleus (which, in turn, implies a greater atomic size). The value n=1 denotes the innermost electron shell of an atom, which corresponds to the lowest energy state or the ground state of an electron. Thus, the principal quantum number, n, cannot have a negative value or be equal to zero. Angular momentum quantum number ℓ The angular or orbital quantum number, describes the sub-shell and gives the magnitude of the orbital angular momentum. A value of the azimuthal quantum number can indicate either an s, p, d, or f subshell which vary in shapes. This value depends on the value of the principal quantum number, i.e., the value of the azimuthal quantum number ranges between 0 and (n-1). ℓ = 0 is called an s orbital, ℓ = 1 is p orbital, ℓ = 2 is d orbital, and ℓ = 3 is f orbital. The value of ℓ ranges from 0 to n − 1 because the first p orbital (ℓ = 1) appears in the second electron shell (n = 2), the first d orbital (ℓ = 2)appears in the third shell (n = 3), and so on. This quantum number specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles. Magnetic Quantum Number mℓ mℓ is associated with the orbital orientation. mℓ describes energy levels available within a sub-shell mℓ yields projection of orbital angular momentum along a specified axis. Value of mℓ ranges from −ℓ to +ℓ, with integer steps between them. The s sub-shell (ℓ=0) contains one orbital, and therefore the mℓ of an electron in an s sub-shell will always be 0. p sub-shell (ℓ=1) contains three orbitals, so mℓ of an electron in a p sub-shell will be −1, 0, or 1. The d sub-shell (ℓ=2) contains five orbitals, with mℓ values of −2, −1, 0, 1, and 2. Spin Quantum Number ms Spin quantum number describes the intrinsic angular momentum of the electron within that orbital and gives the projection of the spin angular momentum (s) along the specified axis. Value of ms ranges from −s to +s. An electron has spin s = ½, consequently ms will be ±½, corresponding to spin and opposite spin Each electron in any individual orbital must have different spins because of the Pauli Exclusion Principle. Thus, an orbital can never contain more than two electrons. The electron spin quantum number is independent of the values of n, ℓ, and mℓ . The value of this number gives insight into the direction in which the electron is spinning and is denoted by the symbol ms. . Thank YOU Lecture: 7 Modern Physics In 1932 Chadwick discover the neutron Atomic Mass Unit One atomic mass unit (1 u) is equal to one twelfth of the mass of the most abundant form of the carbon (12) atom. Atomic mass unit: 1 u = 1.6606 x 10-27 kg = 931.502 MeV/c2. TABLE: Masses in Kilograms, Unified Atomic Mass Units, and 𝑴𝒆𝑽/𝒄𝟐 Mass Objects Kg U 𝑴𝒆𝑽/𝒄𝟐 Electron 9.1094 x 10-31 0.00054858 Proton 1.6726 x 10-27 1.007276 938.27 Neutron 1.6749 x 10-27 1.008665 939.57 1 1𝐻 1.6735 x 10-27 1.007825 938.78 atom 0.511 Properties of Nucleus • Static nuclear properties (Time-independent): Nuclear radius/size, mass, density, charge, spin angular momentum, magnetic dipole moment, electric quadrupole moment. • Dynamic properties (Time-dependent): Self-induced (Radioactive decay), Forced (Nuclear reaction). Nuclear Size: Rutherford’s experiment of α-scattering indicates Atom Nucleus Radius: 10-10m 10-14 to 10-15m The empirical formula of nuclear radius, R = Ro A1/3 where, Ro = 1.3 x 10-15m A => Mass number Nuclear Mass: Mass of the nucleus = Z mp + (A-Z) mn . It is about 99.97% of the mass of atom Nuclear Density: Nuclear density is unbelievably high since is extremely small size and large mass. Nuclear mass The nuclear density, ρ = -----------------------Nuclear volume The mass of 2cm3 of nucleus is 3.7 x 1011 kg Mass of all people of the world = 790,0000000 x 45 kg = 3.56 x 1011kg Nuclear Charge: The charge of the nucleus is due to the charge of the proton which is Ze. Spin angular momentum: Both the proton and neutron like electron, have an intrinsic spin. The spin angular momentum is given by, 𝐿𝑠 = 𝐿𝑠 = 𝑙 𝑙+1 3 ℎ 2 2𝜋 ℎ 2𝜋 where, the quantum number 𝑙, is commonly called the spin, is equal to 1/2. Nuclear magnetic dipole moment: The nuclear magnetic moment is the magnetic moment of an atomic nucleus and arises from the spin of the protons and neutrons. The nuclear magnetic moment varies from isotope to isotope of an element. For a nucleus of which the numbers of protons and of neutrons are both even in its ground state (i.e. lowest energy state), the nuclear spin and magnetic moment are both always zero. In cases with odd numbers of either or both protons and neutrons, the nucleus often has nonzero spin and magnetic moment. According to Dirac’s, the magnetic dipole moment of a spinning proton is given by, ℎ 𝜇= 𝑒2𝜋 2𝑚𝑝 where, mp is the mass of a proton. Nuclear quadrupole moment: In general, a nucleus is considered to be spherical in shape. Electrical quadrupole moment is the measure of deviation of nucleus from spherical symmetry. Electrical quadrupole moment is denoted by symbol “Q“ and is given by 2 𝑄 = 5 𝑧(𝑏2 − 𝑎2) ………. (i) Where z is atomic number and b is radius of nucleus along symmetric axis and a is radius of nucleus along perpendicular to symmetric axis. Now TWO cases arises 1. For nuclei with mass number A < 20, the number of protons and number of neutrons in nucleus almost similar, so charge distribution inside the nucleus will be uniform and shape of nucleus will be a perfect SPHERE. Here b = a and from equation (i), Q = 0. 2. For nuclei with greater mass number A, number of protons and neutrons inside the nucleus are unequal, so charge distribution is non-uniform inside the nucleus . Now there are following 2 possibilities Now there are following 2 possibilities (a) If charge distribution is more along symmetric axis, then in this situation b > a and from equation (i), Q > 0 and Q = +ve. So, there will be some deviation in the nucleus from spherical symmetry and nucleus will be PROLATE SPHEROID as shown in figure. (b) If charge distribution is more along perpendicular to symmetric axis, then in this situation b < a and from equation (i), Q < 0 and Q = -ve. So, there will be some deviation in the nucleus from spherical symmetry and nucleus will be OBLATE SPHEROID as shown in figure. Electrical quadrupole moment (Q) is measured in BARN. 1 BARN = 10-28 m2 Max. Value of Q is found to be for Lutetium (Lu, Z=71, A=176) which is + 7 BARNS. Min. Value of Q is found to be for Antimony (Sb, Z=51, A=123) which is - 1.2 BARNS. As range of values of Q is small, so we can say that deviation in nucleus from spherical symmetry is small. So, our assumption that nucleus is spherical is justified. Radioactive decay: Radioactivity is the property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. The emissions of the most common forms of spontaneous radioactive decay are the alpha (α) particle, the beta (β) particle, the gamma (γ) ray. Radionuclide is an atom that has an unstable nucleus (i.e., an excess of energy, characterized by an excess of protons or neutrons). It will decay (i.e., loss its excess energy) by emitting particles (alpha and beta decay) or photons (gamma rays or Xrays) to reach a stable configuration. Nuclear reaction: A process in which the nucleus of an atom is changed by being split apart or joined with the nucleus of another atom Mass defect: The total mass of a nucleus is always less than the sum of the masses of its separate protons and neutrons. ∆𝑚 = 𝑍𝑚𝑝 + 𝐴 − 𝑍 𝑚𝑛 − 𝑀𝑛𝑢𝑐𝑙𝑒𝑢𝑠 Where has the mass gone? Binding Energy: The minimum energy required to break up a nucleus into its constituent nucleons is known as binding energy. B. E. = Mass defect x c2 = ∆𝑚 ∗ c2 or, 𝑜𝑟, 𝐵. 𝐸. 𝐽 = ∆𝑚 𝑘𝑔 × 𝑐 2 𝑚𝑠 −1 2 𝐵. 𝐸. 𝑀𝑒𝑉 = ∆𝑚 𝑎𝑚𝑢 × 931 Binding Energy per nucleon: The binding energy per nucleon for any nucleus is found by dividing its total binding energy by the total number of nucleons it contains. 𝑇𝑜𝑡𝑎𝑙 𝑏𝑖𝑛𝑑𝑖𝑛𝑔 𝑒𝑛𝑒𝑟𝑔𝑦 𝑜𝑓 𝑎 𝑛𝑢𝑐𝑙𝑒𝑢𝑠 𝐵𝑖𝑛𝑑𝑖𝑛𝑔 𝐸𝑛𝑒𝑟𝑔𝑦 𝑝𝑒𝑟 𝑛𝑢𝑐𝑙𝑒𝑜𝑛 = 𝑇𝑜𝑡𝑎𝑙 𝑛𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑛𝑢𝑐𝑙𝑒𝑜𝑛𝑠 Binding Energy per nucleon: Nuclear force: Nucleus consist of protons and neutrons How does the positive protons and neutral neutrons are kept together in the nucleus? In 1935 Yukawa a Japanese scientist proposed a meson field exists between the protons and neutrons. The particle responsible for nuclear force: π (pi) mesons. π (pi) mesons is 272 times heavier than electron Positive π+ mesons are associated with the force between: p and n Negative π- mesons are associated with the force between: p and p Neutral πo mesons are associated with the force between: n and n Nuclear force: Nucleus consist of protons and neurons How does the positive protons and neutral neutrons are kept together in the nucleus? • Strong nuclear force is an attractive force that attracts among all nucleons (protons and neutrons alike). • Protons attract each other via strong nuclear force at the same time they repel each other via electrostatic force. • Strong nuclear force > electrostatic force. • Neutrons (electrically neutral) only attract other neutrons or protons via strong nuclear force. • Strong nuclear force is a short-range force. It acts only over a very short distance. • It is very strong between two nucleons if they are < 10-15m apart. • It is zero if they are separated by a distance > 10-15 m apart. • If the nuclide contents too fever or too many neutrons relative to the number of protons, the binding of nucleus reduce (nuclide unstable). • Nuclear force is charge independent. It is same for p-p, p-n and n-n interaction. • The energy needed to separate a nucleon from a nucleus is about 8MeV in contrast a few eV is required to separate an electron from an atom. • Nuclear force is limited in range (saturation property). As a result, each nucleon interacts with only a limited number of nucleons around it. Thank YOU Lecture: 8 Modern Physics Nuclear chain reactions Over Christmas vacation in 1938, nuclear chemist Otto Hann observed when Uranium-235 is struck by a thermal neutron it split up and produce neutrons and enormous amount of energy. Physicist Leo Szilard made an important realization: if fission emits neutrons, then neutrons from the fission of one nucleus could cause the fission of another nucleus. Nuclear chain reactions are series of nuclear fissions, which continued by a neutron produced in a preceding fission. For example, on average 21/2 neutrons are released by the fission of each uranium-235 nucleus that absorbs a neutron of moderate/thermal energy. Thermal neutron: A thermal neutron is a free neutron with a kinetic energy of about 0.025 eV (about 4.0×10−21 J, hence a speed of 2.19 km/s), at a temperature of about 290 K (17 °C or 62 °F), Source: Internet Multiplication factor, k: The condition for a chain reaction is usually expressed in terms of a multiplication factor, k, which is defined as the ratio of the number of fissions produced in one step (neutron generation) in the chain to the number of fissions in the preceding generation. 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑛𝑒𝑢𝑡𝑟𝑜𝑛𝑠 𝑝𝑟𝑜𝑑𝑢𝑐𝑒𝑑 𝑖𝑛 𝑎𝑛𝑦 𝑔𝑒𝑛𝑒𝑟𝑎𝑡𝑖𝑜𝑛 𝑘= 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑛𝑒𝑢𝑡𝑟𝑜𝑛 𝑝𝑟𝑜𝑑𝑢𝑐𝑒𝑑 𝑖𝑛 𝑡ℎ𝑒 𝑝𝑟𝑒𝑐𝑒𝑑𝑖𝑛𝑔 𝑔𝑒𝑛𝑒𝑟𝑎𝑡𝑖𝑜𝑛 If k is less than unity, a chain reaction cannot be sustained. If k = 1, a steady-state chain reaction can be maintained; and if k is greater than 1, the number of fissions increases at each step, resulting in a divergent chain reaction. Although two to three neutrons are produced for every fission, not all of these neutrons are available for continuing the fission reaction. If the conditions are such that the neutrons are lost at a faster rate than they are formed by fission, the chain reaction will not be self-sustaining. At the point where the chain reaction can become self-sustaining, this is referred to as critical mass. Critical mass: In nuclear physics, critical mass is the minimum amount of a given fissile material necessary to achieve a self-sustaining chain reaction. It depends on several factors, including the kind of fissile material used, its density and purity, temperature, the composition and geometry of the surrounding. The critical mass of a bare sphere of uranium-235 at normal density is approximately 47 kg (104 pounds); for plutonium-239, critical mass is approximately 10 kg (22 pounds). By surrounding the fissionable material with a suitable neutron reflector, the loss of neutrons can reduced and the critical mass can also be reduced. By using a neutron reflector, only about 11 pounds (5 kilograms) of nearly pure plutonium 239 and about 33 pounds (15 kilograms) uranium 235 is needed to achieve critical mass. Neutron reflector: A layer of material immediately surrounding a fissionable material that reflects back the neutrons into the core. Without reflector many neutrons that would otherwise escape. The returned neutrons can then cause more fissions and improve the neutron economy of the chain reaction. A reflector made of a light material like graphite or beryllium will also serve as a neutron moderator reducing neutron kinetic energy, while a heavy material like lead will have less effect on neutron velocity. Critical size: The actual size of fissionable material which allows the escape of neutrons to such an way that at least one neutron is positively left behind per fission reaction is known as critical size. The critical size must at least include enough fissionable material to reach critical mass. If the size of the fissionable material is less than a certain minimum, too many fission neutrons escape through its surface and the chain reaction is not sustained. In order to minimize the losses through the surface, the area of the surface ‘S’ (through which fission neutrons escape) must be made as small as possible in comparison to the volume ‘V’ (in which fission take place). Larger the size of the fissionable material, smaller the surface to volume (S/V) ratio becomes. Surface area of a sphere, S = 4πr2 4 Volume of a sphere, V = πr3 3 Therefore, S/V = 3/r = 6/d, i. e., inversely proportional to size of the material If, d = 0.2 cm ; S/V = 30 Again, if, d = 20 cm (100 times larger); S/V = 0.3 (100 times smaller) Bare sphere critical masses and size U-233 => 15 kg, 11 cm of diameter U-235 => 52 kg, 17 cm of diameter Pu-239 => 10 kg, 9.9 cm of diameter Nuclear Fission: The process by which a heavy nucleus is split up into two fragments of roughly equal mass nuclei is known as nuclear fission. Source: Internet + Energy +Energy Nuclear Fission: + Energy +Energy + Energy + Energy 1.0087amu + 235.0457amu = 140.9177amu + 91.8859amu + 3 x 1.0087amu + Energy Mass Difference = 236.0544amu – 235.8297amu = 0.2247amu Therefore released energy = 210MeV Nuclear Fusion: The process by which two small nuclei fused together to form a relatively big nucleus is known as nuclear fusion. Energy 2.0147amu + 2.0147amu → 4.0039amu + Energy Mass Difference = 4.0294amu –4.0039amu = 0.0255amu Therefore released energy = 24MeV Second World War Germany, Italy, Japan, Rassia . . . . . 1 September, 1939, Germany attacked the Poland 3 September, 1939, England and France announced war against Germany Germany, Japan, Italy, Romania…. USA, Rassia, England, France, Canada…. 7Dec, 1941, Japan attacked PEARL HARBOR, then USA joined German surrendered: 8 May 1945 Hiroshima: 6 Aug 1945 Nagasaki: 9 Aug 1945 Japan surrendered 14 Aug 1945 Germany Heisenberg 1939 U split 1943 Lab Destroyed 1945 May 3, Heisenberg arrested and migrated to England USA J. Robert Oppenheimer Einstein Letter to Franklin D. Roosevelt 1942 Manhattan Project Within 1945, July; 3 atom bomb At 5:30 a.m. on July 16, 1945, Trinity Test: Gadget. Total Expenditure: 2 Billion Dollar On August 6, 1945, at about 8:15am local time, the US aircraft Enola Gay dropped an untested uranium-235 gun-assembly bomb nicknamed "Little Boy" over Hiroshima. Hiroshima was immediately flattened. The resulting explosion killed 70,000 people instantly; by December 1945, the death toll had risen to 140,000. Within next few years about more than 200,000 people died. The radius of total destruction was reportedly 1.6km. Source: Internet On August 9, 1945, at about 10:58am local time, Boeing B-29 dropped a plutonium-239 implosion type bomb nicknamed “Fat Man" over Nagasaki. Source: Internet Little Boy: A Gun-Type Bomb The Little boy was 3.0 m long, 0.71 m in diameter, and weighed 4,400 kg (64Kg of U) and blast yield was 15 KTon of TNT (63 x 1012 J). The Little Boy designed as a gun-type, that fired one mass of uranium 235 at another mass of uranium 235, thus creating a supercritical mass. Once the two pieces of uranium are brought together, the initiator introduces a burst of neutrons and the chain reaction begins, continuing until the uranium become finished. Fat Man: Implosion-Type Bomb The device was 3.3 m long, 1.5 m in diameter, and weighed 4,600 kg (6.4 Kg of Pu) and blast yield was 21 KTon of TNT (88 x 1012 J). “Fat Man” was an implosion-type device using plutonium 239. A subcritical sphere of plutonium was placed in the center of a hollow sphere of high explosive. Thirty-two pairs of detonators located on the surface of the high explosive were fired simultaneously to produce a powerful inward pressure on the core, squeezing it and increasing its density, resulting in a supercritical condition and a nuclear initiation. The first was the "Gadget“. Known as Trinity test on July 16, 1945. Source: Internet Tsutomu Yamaguchi was a Japanese marine engineer and a survivor of both the Hiroshima and Nagasaki atomic bombings during World War II. Nuclear Power Reactor Source: Internet Source: Internet Nuclear Power Reactor A nuclear reactor produces and controls the release of energy from splitting the atoms of certain elements. In a nuclear power reactor, the energy released is used as heat to make steam to generate electricity. The principles for using nuclear power to produce electricity are the same for most types of electric power plant. The following are the stages/components in the working of a power reactor. Enrichment The percentage of U in the naturally occurring uranium is only about 0.7%. In a nuclear reactor, the percentage of U-235 is increased by a process called enrichment. This is usually achieved either by gaseous diffusion or by a centrifuge. In the diffusion process, natural uranium, which is first converted to gaseous uranium hexafluoride (UF) is passed through a series of semiporous membranes which permit passage of the gas that contains the lighter U-235 but not the component containing the heavier isotope U-238. The technique is continued/repeated until achieve the desired concentration of about 4 to 5% of U-235. In the centrifuge technique the natural UF is spun at high speed which separates the heavier and the lighter components as they are subjected to different centrifugal forces. Fuel The fuel (Uranium U235, U238, Thorium Th232, Plutonium Pu239, Pu240, Pu241 etc.) are used in running the reactor. Usually pellets of uranium oxide (UO2) are arranged in tubes to form fuel rods. The rods are arranged into fuel assemblies in the reactor core. In a 1000 MW class Pressurized Water Reactor (PWR) there might be 51,000 fuel rods with over 18 million pellets. The mass of the fissile material required to sustain chain reaction is said to be critical mass. If a reactor cannot sustain chain reaction, it is called sub-critical. On the other hand if the chain reaction proceeds uncontrolled, as in an atom bomb, the reactor is called supercritical. Control rod These are made with neutron-absorbing material such as cadmium or boron, and are inserted or withdrawn from the core to control the rate of reaction, or to halt it to make sure that the reactor does not become supercritical. In nuclear industry cadmium is commonly used as a thermal neutron absorber due to very high neutron absorption cross-section of 113Cd. There is a cadmium cut-off energy for the absorption. Only neutrons of kinetic energy below the cadmium cut-off energy (~0.5 eV) are strongly absorbed by 113Cd. Therefore, cadmium is widely used to absorb thermal neutrons in nuclear reactor. In general, the absorption cross-section is an effective area that quantifies the likelihood of certain interaction between an incident object and a target object. Coolant A variety of substances, including light water, heavy water, air, carbon dioxide, helium, liquid sodium etc., have been used as coolants. Such substances are, in general, good conductors of heat, and they serve to carry the thermal energy from the fuel to the steam-generating equipment of the nuclear power plant. Moderator In many cases, the same substance functions as both coolant and moderator, as in the case of light and heavy water. Graphite and beryllium are also used as moderator. The moderator slows the fast (high-energy) neutrons emitted during fission to energies at which they are more likely to induce fission. In doing so, the moderator helps initiate and sustain a fission chain reaction. Neutrons reflector A reflector is a region of unfueled material surrounding the core. Its function is to scatter neutrons that leak from the core, thereby returning some of them back into the core. The reactor allows for a smaller core size. In addition, reflectors “smooth out” the power density by utilizing neutrons that would otherwise leak out through fissioning within fuel material located near the core’s outer region. Two types of reactor Boiling Water Reactor (BWR) In a boiling water reactor, water in pipes circulates inside the core. The water gets heated, generates steam which is then used to drive turbines. As the water enters the core, there is a possibility of its becoming radioactive. Further, in case of a rapture of the pipe due to extreme heat, it could lead to accidents. Pressurized Water Reactor (PWR) In these reactors water is extracted in two steps. The primary coolant, as in the case of BWR circulates inside the core. However, the water circulates under great pressure so that it does not become steam. The heat is transferred through a heat exchanger to a secondary coolant, which may be used to drive a turbine. As the secondary coolant does not enter the core, it does not become radioactive. Source: Internet Thank YOU Lecture: 9 Modern Physics The fusion of nuclei can take place in several different reaction sequences, the most common of which, the proton-proton cycle as suggested by Hans Bethe. A neutrino is a subatomic particle has no electrical charge and a very small mass, which might even be zero. p-p cycle 1. 1H1 + 1H1 1H2 + e+ + neutrino 2. 1H2 + 1H1 2He3 + Gamma 3. 2He3 + 2He3 2He4 + 1H1 +1H1+ 24.7MeV = 4 x 10-12 J Which is a better option to generate power, nuclear fission or fusion? Fusion is much better than fission in a number of ways: Firstly, nuclear fusion requires less fuel than fission. Secondly, fusion is carried out by using deuterium (an isotope of hydrogen) as fuel, which is quite abundant in nature. In contrast, the fuel necessary for fission (uranium, plutonium or thorium) is very hard to get, and very expensive! Thirdly, unlike fission, nuclear fusion does not produce any radioactive waste; it only produces helium atoms as a byproduct, which we can actually use to our benefit in various ways. Fourthly, Since fusion doesn’t produce chain reactions it should be easy to control. So, if fusion is so great, and better than fission in so many respects, why aren’t we using fusion to generate power already? Difficulties against using nuclear fusion When hydrogen atoms fuse, the nuclei must come together. However, the protons in each nucleus will tend to repel each other because they have the same charge. High temperature: The high temperature gives the hydrogen atoms enough energy to overcome the electrical repulsion between the protons. Fusion requires temperatures about 100 million Kelvin (approximately six times hotter than the sun's core). At these temperatures, hydrogen is a plasma, not a gas. Plasma is a high-energy state of matter in which all the electrons are stripped from atoms and move freely about. The sun achieves these temperatures by its large mass and the force of gravity compressing this mass in the core. High pressure and density: High pressure squeezes the hydrogen atoms together. They must be within 1x10-15 m of each other to fuse. The sun uses its mass and the force of gravity to squeeze hydrogen atoms together in its core. This high density ensure that collisions among protons are frequent. There are two ways to achieve the temperatures and pressures necessary for hydrogen fusion to take place: Magnetic confinement uses magnetic and electric fields to heat and squeeze the hydrogen plasma. The ITER (the International Thermonuclear Experimental Reactor) project in France is using this method. Inertial confinement uses laser beams or ion beams to heat and squeeze the hydrogen plasma. Scientists are studying this experimental approach at the National Ignition Facility of Lawrence Livermore Laboratory in the United States. Let's look at magnetic confinement first. Here's how it would work: Microwaves, electricity and neutral particle (they do not leave tracks of ionized particles or curve in magnetic fields) beams from accelerators heat a stream of hydrogen gas. This heating turns the gas into plasma. This plasma gets squeezed by super-conducting magnets, thereby allowing fusion to occur. The most efficient shape for the magnetically confined plasma is a donut shape (toroid) called TOKAMAK. Let's take a closer look at the ITER fusion reactor to see how magnetic confinement works. ITER ("The Way" in Latin) is one of the most ambitious energy projects in the world today. In southern France, 35 nations (The ITER Members China, the European Union, India, Japan, Korea, Russia and the United States) are collaborating to build the world's largest tokamak. ITER project Inside Tokamak (Vacuum chamber) Construction/The main parts of the tokamak reactor are: Vacuum vessel - holds the plasma and keeps the reaction chamber in a vacuum Neutral beam injector (ion cyclotron system) - injects particle beams from the accelerator into the fusion fuel to help the fuel to critical temperature to make plasma. Magnetic field coils (poloidal, toroidal) - super-conducting magnets that confine shape and contain the plasma using magnetic fields. Central solenoid/Transformers - supply electricity to the magnetic field coils. Cryostat (cooling equipment) - cool the magnets, provide a supercool vacuum environment. Blanket modules - made of lithium; absorb heat and absorb high-energy neutrons from the fusion reaction to transfer their energy to heat water. Divertors - exhaust the helium products of the fusion reaction. Basic working process 1. The fusion reactor will heat a stream of deuterium and tritium fuel to form high-temperature plasma. It will squeeze the plasma so that fusion can take place. 2. The lithium blankets outside the plasma reaction chamber will absorb highenergy neutrons from the fusion reaction also get heated. 3. The heat will be transferred by a water-cooling loop to a heat exchanger to make steam. 4. The steam will drive electrical turbines to produce electricity. 5. The steam will be condensed back into water to absorb more heat from the reactor in the heat exchanger. Hydrogen Bomb On November 1, 1952, the United States detonated a 10.4-megaton hydrogen device in the Pacific on the Marshall Islands. The test, code-named "Mike," was the first successful test. Those who had witnessed atomic tests were stunned by the blast. The cloud, when it had reached its furthest extent, was about 100 miles wide and 25 miles high. The fireball reached about 3.25 mile in diameter. Elugelab, the island on which Mike’s detonation took place, was indeed vaporized, leaving a crater 2km in diameter and 50m deep. The MIKE Test (Operation Ivy) Fusion Reactors: Inertial Confinement The National Ignition Facility (NIF) at Lawrence Livermore Laboratory, USA is experimenting with using laser beams to induce fusion. In the NIF device, about 192 laser beams will focus on single point in a 10-meter-diameter target chamber called a hohlraum. A hohlraum is a cavity whose walls are in radiative equilibrium (Radiative equilibrium is the condition where the total thermal radiation leaving an object is equal to the total thermal radiation entering it). At the focal point inside the target chamber, there will be a pea-sized pellet of deuterium-tritium encased in a small, plastic cylinder. The power from the lasers (1.8 million joules) will heat the cylinder which convert the pellet into plasma and compress (rocket like blowoff) it until fusion occurs. The fusion reaction will be short-lived, about one-millionth of a second, but will yield about 50 times more energy than is needed to initiate the fusion reaction. Like the magnetic-confinement fusion reactor, the heat from inertialconfinement fusion will be passed to a heat exchanger to make steam for producing electricity. Flibe is a molten salt made from a mixture of lithium fluoride (LiF) and beryllium fluoride (BeF2). Hyperspectral imagery (HSI) has several image bands captured at distinct wavelengths in the electromagnetic spectrum. Figure: Laser Inertial Confinement Fusion Reactor conceived at Lawrence Livermore Laboratory. During the final part, the fuel core reaches around 1000 times of its initial density (about 20 times the density of lead) and about 107 to 108 0 C. Nuclear models The aim of nuclear models is to understand how neutrons and protons form a nucleus. The liquid drop model The liquid drop model of the nucleus, proposed by Bohr and derived by Von Weizsacker in 1935, was one of the earliest phenomenological successes constructed to account for the binding energy of a nucleus. They marked few similarities between a nucleus and a liquid drop, these are follows: (i) In the stable state, a nucleus is supposed to be spherical in shape, just as a liquid drop is spherical due to the symmetrical surface tension forces. (ii) Just as the force of surface tension acts on the surface of the liquid drop, there is a potential barrier acts at the surface of the nucleus. (iii) The density of a liquid drop is independent of its volume. Similarly, the density of the nucleus is also almost independent of its volume. (iv) The intermolecular forces in a liquid are short range forces. The molecules in a liquid drop interact only with their immediate neighbors. Similarly, the nucleons in the nucleus also interact only with their immediate neighbors. That means, the nuclear forces are also sort rang forces. This leads to the saturation of nuclear forces and a constant binding energy per nucleon. (V) When the temperature of the liquid is raised, the molecules from a liquid drop evaporate due to their increased energy of thermal agitation. Similarly, when a nucleus is bombarded with nuclear projectiles it gains energy and a compound nucleus is formed. But the compound nucleus emits nuclear radiation almost immediately. (vi) When a small drop of liquid allow to oscillate, it breaks up into two similar drops of equal size. Similarly, in nuclear fission the nucleus breaks up into two nuclei of almost equal mass numbers. (vii) Two small liquid drop make a large drop by combining together. Similarly two deuterium produce helium by fusion process. Semi-empirical mass formula The liquid drop model can be used to obtain an expression for the binding energy of the nucleus. We shall start by assuming that the energy associated with each nucleon-nucleon bond has some value U. Volume energy: Since each bond energy is shared by two nucleons, the binding energy associated with each nucleon 1/2U. When an assembly of spheres of the same size is packed together in the smallest possible volume, each interior sphere has 12 other spheres in contact with it. Hence each interior nucleon in a nucleus has a binding energy of (12) (1/2U) or 6U. if all the A nucleons in a nucleus could be considered to be in the interior of the nucleus, the total binding energy of the nucleus would be Ev = (6U)(A) = 6 AU = a1 A …………… (1) (Volume energy) The energy Ev is called volume energy of a nucleus and is proportional it A. Thank YOU Lecture: 10 Modern Physics Semi-empirical mass formula The liquid drop model can be used to obtain an expression for the binding energy of the nucleus. We shall start by assuming that the energy associated with each nucleon-nucleon bond has some value U. Volume energy: Since each bond energy is shared by two nucleons, the binding energy associated with each nucleon 1/2U. When an assembly of spheres of the same size is packed together in the smallest possible volume, each interior sphere has 12 other spheres in contact with it. Hence each interior nucleon in a nucleus has a binding energy of (12) (1/2U) or 6U. if all the A nucleons in a nucleus could be considered to be in the interior of the nucleus, the total binding energy of the nucleus would be Ev = (6U)(A) = 6 AU = a1 A …………… (1) (Volume energy) The energy Ev is called volume energy of a nucleus and is proportional it A. Surface energy: Actually not all the nucleons are within the interior the nucleus. Some nucleons are on the surface of the nucleus and therefore have fewer than 12 neighbors. The number of such nucleons depends on the surface area of the nucleus. A nucleus of radius R has an area of 4πR2 = 4π𝑅02 𝐴2/3 Hence the number of nucleons with fewer than the maximum number of bonds is proportional to A2/3. Thus the total binding energy is reduced by Es = -a2A2/3 …………..(2) (Surface energy) It is most significant for the lighter nuclei since a greater fraction of their nucleons are on the surface. Because natural system always tend to evolve toward configurations of minimum potential energy, nuclei tend toward configurations of maximum binding energy. Hence a nucleus should exhibit the same surface-tension effects as a liquid drop. Therefore, in the absence of external forces it should be spherical, since a sphere has the least surface area for a given volume. Coulomb energy: The electrostatic force of repulsion between two protons (a pair of protons) in a nucleus also decreases the binding energy of the nucleus. The potential energy of a pair of protons separated by a distance r is given by 𝑒2 𝑉=− 4𝜋𝜀0 𝑟 Since there are Z(Z-1)/2 pairs of protons in a nucleus, coulomb energy, 𝑍(𝑍 − 1) 𝑍 𝑍 − 1 𝑒2 1 𝐸𝑐 = .𝑉 = − 2 8𝜋𝜀0 𝑟 where 1 𝑟 𝑎𝑣 is the value of 1 𝑟 averaged over all proton pairs. If the protons are uniformly distributed throughout a nucleus of radius R, and hence to 1 𝐴1/3 𝑎𝑣 1 is 𝑟 𝑎𝑣 proportional to 1 𝑅 , so that 𝑬𝒄 = −𝒂𝟑 𝒁(𝒁−𝟏) ………… (3) 𝑨𝟏/𝟑 (Coulomb energy) The Coulomb energy is negative because it arises from an effect that opposes nuclear stability. The total binding energy of the nucleus according to liquid drop model ought to be the sum of its volume, surface and coulomb energies: Eb = Ev + Es + Ec = a1A – a2A2/3 - 𝒂𝟑 𝒁(𝒁−𝟏) 𝑨𝟏/𝟑 The binding energy per nucleon is therefore, 𝑬𝒃 𝑨 = 𝑩𝑬 𝑨 = 𝒂𝟏 − 𝒂𝟐 𝑨𝟏/𝟑 − 𝒂𝟑 𝒁(𝒁−𝟏) … 𝑨𝟒/𝟑 … … … (4) All terms of eqn. 4 is plotted in Fig. 1 versus A. Since the theoretical curve is fit so well with the Semiempirical mass formula means that the analogy between a nucleus and a liquid drop has some validity at least. Fig. 1 The Shell Model The shell model assumes that the energy structure (energy levels) of the nucleons in a nucleus is similar to that of electrons in an atom. According to this model, the protons and neutrons are grouped in shells in the nucleus in a manner similar to grouping of electrons in various shells outside the nucleus. The shells are regarded as "filled“ when they contain a specific number of protons or neutrons or both. It should be remembered that the case is not so simple as it appears. In the extranuclear shells only one type of particles (i.e., electrons) is to be arranged in different shells and Pauli's exclusion principle is applied. In the case of the nucleus there are two types of particles, protons and neutrons and the shell arrangement is only empirical and based upon the study of the stability and interactions of the nuclides which are known. Nuclei that have 2, 8, 20, 28, 50, 82 and 126 neutrons or protons are more abundant than other nuclei of similar mass number, suggesting that their structures are more stable. Many evidence also points up the significance in nuclear structure of the numbers 2, 8, 20, 28, 50, 82 and 126. These numbers known as magic numbers. The main points in favour of this inference are: (i) The inert gases with-closed electron shells exhibit a high degree of chemical stability. Similarly, nuclides whose nuclei contain a magic number of nucleons exhibit more than average stability. (ii) Nuclides of isotopes of elements having an isotopic abundance greater than 60% contain magic numbers. (iii) The stable end product of the three main radioactive series (viz, the uranium, actinium and thorium series) is 82Pb208 which contains 82 protons and 126 neutrons. Thus, 82Pb208 is the most stable isotope. This shows that the number 82 and 126 indicate stability. (iv) Tin (50Sn) has ten stable isotopes, while (20Ca) has six stable isotopes. So, elements with Z = 50, 20 are usually more stable. (v) 8O17, 36Kr87 and 54Xe137 isotopes spontaneously emit neutrons. For these isotopes, the number of neutrons are 9, 51 and 83 respectively which can be written as 8+1, 50+1 and 82+1. If this loosely bound neutron is interpreted as a valency neutron, then the neutron numbers 8, 50 and 82 represent greater stability than other neutron numbers. 𝑬𝒃 𝑨 Asymmetry energy: = 𝑩𝑬 𝑨 𝒂 𝟐 = 𝒂𝟏 − 𝑨𝟏/𝟑 − 𝒂𝟑 𝒁(𝒁−𝟏) … 𝑨𝟒/𝟑 … … … (4) The binding energy formula as given by eqn. 4 can be improved by two effects that do not fit the simple liquid drop model, but which can be readily explained in terms of a model that take into account nuclear energy levels (shell model). One of these effects occur when the neutrons in a nucleus outnumber the protons or vice versa, which means that higher energy levels have to be occupied than would be the case if N and Z are equal. Let the uppermost neutron and proton energy levels as shown in Fig. 1 which have the same energy spacing 𝜺. In order to produce a neutron excess, say, N-Z = 8 without changing 1 A, 2(N- Z) = 4 protons converted to neutrons in an original nucleus in which N = Z. The new neutrons would occupy levels higher in energy by 2 𝜀 = 4 (𝜀 /2). Each shifted proton (new neutron) raised in energy by 1 (𝑁 2 𝜀 − 𝑍) 2 . Fig. 1 The total raised in energy, ΔE = number of new neutron x increased energy by each neutron = 1 (𝑁 2 − 𝑍) 1 (𝑁 2 𝜀 𝜀 − 𝑍) 2 = 8 𝑁 − 𝑍 2 The same formula will hold if Z> N, since(N-Z)2 is always positive. Now, N= A-Z, therefore (N-Z)2 = (A-2Z)2, and 𝜀 ∆𝐸 = 𝐴 − 2𝑍 2 8 The greater the number of nucleons in a nucleus, the smaller is the energy level spacing 𝜀, with 𝜀 proportional to 1/A. The departure from N=Z introduces an asymmetry N-Z, which results in a decrease in binding energy. This means that the asymmetry energy Ea due to the difference between N and Z can be expressed as 𝐸𝑎 = −∆𝐸 = 𝐴−2𝑍 2 −𝑎4 …………………(4) 𝐴 (Asymmetry energy) The asymmetry energy is negative because it reduces the binding energy of the nucleus. Pairing energy: The final correction term arises from the tendency of protons and neutrons to occur in pairs. Even-even nuclei are the most stable and hence have higher binding energies than would otherwise be expected. Thus even-even nuclei like 2He4, 6C12, 16 8O appear as peaks on the empirical curve of binding energy per nucleon. On the other hand, odd-odd nuclei have both unpaired protons and neutrons and have relatively low binding energies. The pairing energy Ep, is positive for even-even nuclei, 0 for odd-even and even-odd nuclei, and negative for odd-odd nuclei and seems to vary with A as A-3/4 . Hence 1 𝐸𝑝 = ±, 0 𝑎5 𝐴3/4 … … … … …(5) (Pairing energy) Total Binding Energy The final expression for the binding energy of a nucleus of atomic number Z and mass number A was first obtained by C. F. Von Weizsacker in 1935 and is known as Weizsacker’s semi-empirical mass formula. This Is given by 𝐸𝑏 = 𝑎1 𝐴 − 2 𝑎2 𝐴3 − 𝑎3 𝑍 𝑍−1 1 𝐴3 − 𝑎4 𝐴 − 2𝑍 𝐴 The best values of the constants, expressed in MeV, are a1 = 15.76, a2 = 17.81, a3 = 0.711, a4 = 23.702 and a5 = 34 2 (±, 0)𝑎5 1 𝐴3/4 Radioactivity • Properties of ∝ - particles, 𝜷- particles and 𝜸- rays • Radioactive transformation • Law governing radioactive disintegration/ Decay law 𝒅𝑵 ∝𝑵 𝒅𝒕 = 𝑵𝟎 𝒆−λ𝒕 − 𝑵 • Half life period 𝟎. 𝟔𝟗𝟑 𝑻𝟏/𝟐 = λ • Average life period (or Mean life) of a radioelement 𝟏 𝑻𝒂 = λ Average life period (or Mean life) of a radioelement Since the radioactive atoms are constantly disintegrating one after another, the life of every atom is different. The atoms which disintegrate earlier have very short life whereas others which disintegrate at the end have a very long time. Thus, the possible time of existence of a radioactive atom may vary from zero to infinity. The average life period (or mean life ) of a radioactive clement is defined as the ratio of the sum of lifetime of all the radioactive atoms to the total number of such atoms in it. Let N0 be the total number of radioactive atoms in the beginning at t= 0. Let N be the number of atoms at that element after a time t. Now let the dN atoms disintegrate between t and (t + dt). Since dt is very small, we may assume without serious error that all these atoms have lived for a time t. Hence the total lifetime of these dN atoms = (-dN)t. The possible life of any atom varies from 0 to ∝, the total life period of all atoms is given by ∝ = −𝑑𝑁 𝑡 0 Therefore, Average life period, 𝑆𝑢𝑚 𝑜𝑓 𝑡ℎ𝑒 𝑙𝑖𝑓𝑒 𝑝𝑒𝑟𝑖𝑜𝑑 𝑜𝑓 𝑎𝑙𝑙 𝑡ℎ𝑒 𝑎𝑡𝑜𝑚𝑠 𝑇𝑎 = 𝑇𝑜𝑡𝑎𝑙 𝑛𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑎𝑡𝑜𝑚𝑠 ∝ 0 𝑢𝑣𝑑𝑥 = 𝑢 𝑣𝑑𝑥 − 𝑑𝑢 𝑑𝑥 −𝑑𝑁 𝑡 = 𝑁0 ∝ λ 𝑁0 𝑒 −λ𝑡 𝑡𝑑𝑡 0 = 𝑁0 𝑣𝑑𝑥 𝑑𝑥 ∝ −𝑑𝑁 ∝𝑁 𝑑𝑡 𝑑𝑁 = −λ 𝑁 𝑑𝑡 = −λ𝑁0 𝑒 −λ𝑡 𝑑𝑡 𝑡𝑒 −λ𝑡 𝑑𝑡 =λ 0 = λ 𝑡. 𝑒 −λ𝑡 −λ − 1. 𝑒 −λ𝑡 −λ Therefore, Average life period, 𝑇𝑎 = 1 λ 𝑑𝑡 0 1 −λ𝑡 ∝ −λ𝑡 = −𝑡𝑒 − 𝑒 λ 0 =− 0+0 − 0+ ∝ 1 λ Thank YOU