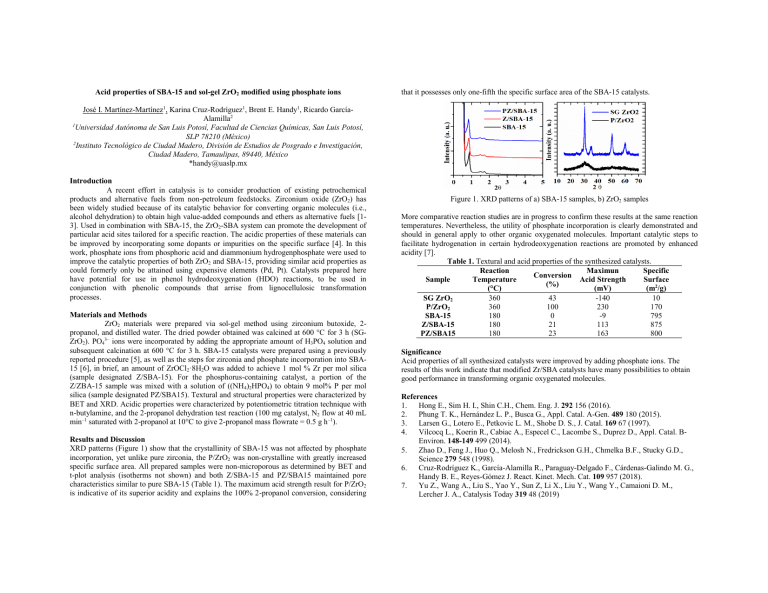
Acid properties of SBA-15 and sol-gel ZrO2 modified using phosphate ions that it possesses only one-fifth the specific surface area of the SBA-15 catalysts. José I. Martínez-Martínez1, Karina Cruz-Rodríguez1, Brent E. Handy1, Ricardo GarcíaAlamilla2 1 Universidad Autónoma de San Luis Potosí, Facultad de Ciencias Químicas, San Luis Potosí, SLP 78210 (México) 2 Instituto Tecnológico de Ciudad Madero, División de Estudios de Posgrado e Investigación, Ciudad Madero, Tamaulipas, 89440, México *handy@uaslp.mx Introduction A recent effort in catalysis is to consider production of existing petrochemical products and alternative fuels from non-petroleum feedstocks. Zirconium oxide (ZrO2) has been widely studied because of its catalytic behavior for converting organic molecules (i.e., alcohol dehydration) to obtain high value-added compounds and ethers as alternative fuels [13]. Used in combination with SBA-15, the ZrO2-SBA system can promote the development of particular acid sites tailored for a specific reaction. The acidic properties of these materials can be improved by incorporating some dopants or impurities on the specific surface [4]. In this work, phosphate ions from phosphoric acid and diammonium hydrogenphosphate were used to improve the catalytic properties of both ZrO2 and SBA-15, providing similar acid properties as could formerly only be attained using expensive elements (Pd, Pt). Catalysts prepared here have potential for use in phenol hydrodeoxygenation (HDO) reactions, to be used in conjunction with phenolic compounds that arrise from lignocellulosic transformation processes. Materials and Methods ZrO2 materials were prepared via sol-gel method using zirconium butoxide, 2propanol, and distilled water. The dried powder obtained was calcined at 600 °C for 3 h (SGZrO2). PO43– ions were incorporated by adding the appropriate amount of H3PO4 solution and subsequent calcination at 600 °C for 3 h. SBA-15 catalysts were prepared using a previously reported procedure [5], as well as the steps for zirconia and phosphate incorporation into SBA15 [6], in brief, an amount of ZrOCl2·8H2O was added to achieve 1 mol % Zr per mol silica (sample designated Z/SBA-15). For the phosphorus-containing catalyst, a portion of the Z/ZBA-15 sample was mixed with a solution of ((NH4)2HPO4) to obtain 9 mol% P per mol silica (sample designated PZ/SBA15). Textural and structural properties were characterized by BET and XRD. Acidic properties were characterized by potentiometric titration technique with n-butylamine, and the 2-propanol dehydration test reaction (100 mg catalyst, N2 flow at 40 mL min–1 saturated with 2-propanol at 10°C to give 2-propanol mass flowrate = 0.5 g h–1). Results and Discussion XRD patterns (Figure 1) show that the crystallinity of SBA-15 was not affected by phosphate incorporation, yet unlike pure zirconia, the P/ZrO2 was non-crystalline with greatly increased specific surface area. All prepared samples were non-microporous as determined by BET and t-plot analysis (isotherms not shown) and both Z/SBA-15 and PZ/SBA15 maintained pore characteristics similar to pure SBA-15 (Table 1). The maximum acid strength result for P/ZrO2 is indicative of its superior acidity and explains the 100% 2-propanol conversion, considering Figure 1. XRD patterns of a) SBA-15 samples, b) ZrO2 samples More comparative reaction studies are in progress to confirm these results at the same reaction temperatures. Nevertheless, the utility of phosphate incorporation is clearly demonstrated and should in general apply to other organic oxygenated molecules. Important catalytic steps to facilitate hydrogenation in certain hydrodeoxygenation reactions are promoted by enhanced acidity [7]. Table 1. Textural and acid properties of the synthesized catalysts. Reaction Maximun Specific Conversion Sample Temperature Acid Strength Surface (%) (°C) (mV) (m2/g) SG ZrO2 360 43 -140 10 P/ZrO2 360 100 230 170 SBA-15 180 0 -9 795 Z/SBA-15 180 21 113 875 PZ/SBA15 180 23 163 800 Significance Acid properties of all synthesized catalysts were improved by adding phosphate ions. The results of this work indicate that modified Zr/SBA catalysts have many possibilities to obtain good performance in transforming organic oxygenated molecules. References 1. Hong E., Sim H. I., Shin C.H., Chem. Eng. J. 292 156 (2016). 2. Phung T. K., Hernández L. P., Busca G., Appl. Catal. A-Gen. 489 180 (2015). 3. Larsen G., Lotero E., Petkovic L. M., Shobe D. S., J. Catal. 169 67 (1997). 4. Vilcocq L., Koerin R., Cabiac A., Especel C., Lacombe S., Duprez D., Appl. Catal. BEnviron. 148-149 499 (2014). 5. Zhao D., Feng J., Huo Q., Melosh N., Fredrickson G.H., Chmelka B.F., Stucky G.D., Science 279 548 (1998). 6. Cruz-Rodríguez K., García-Alamilla R., Paraguay-Delgado F., Cárdenas-Galindo M. G., Handy B. E., Reyes-Gómez J. React. Kinet. Mech. Cat. 109 957 (2018). 7. Yu Z., Wang A., Liu S., Yao Y., Sun Z, Li X., Liu Y., Wang Y., Camaioni D. M., Lercher J. A., Catalysis Today 319 48 (2019)