Catalytic oxidation of CO over CuxCe1-xO2-x/SBA
advertisement

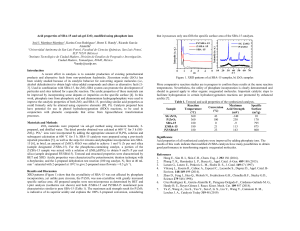
CATALYTIC OXIDATION OF CO OVER CUXCE1-XO2-X/SBA-15/FECRAL MONOLITHIC CATALYSTS Fuzhen Zhao, Shengfu Ji*, Zhenfeng Li, Chengyue Li State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China * Corresponding author: Fax: +86-10-64419619, jisf@mail.buct.edu.cn Recently, catalytic oxidation of CO has received much attention due to its importance in pollution control derives for vehicle exhaust, indoor air cleaning, fuel cells, and minimizing the CO build-up in CO2 lasers. Most of the conventional oxidation catalysts are noble metal or transition metal oxide catalysts. These catalyst pellets made the fixed-bed reactors to have high pressure drops and high gradient of temperature and thus depressed the activity of the catalysts. Nowadays, novel monolithic structured catalysts, especially with the FeCrAl alloy foils as the catalyst support, have increasingly drawn the attention of researchers. The monolithic catalysts have distinct advantages such as the much lower pressure drops, the lower capacity of mass and heat transfer and the higher resistance to thermal shocks. In this study, a series of CuxCe1-xO2-x(x = 0-1)-based metal monolithic catalysts on supports made of the FeCrAl alloy foils were prepared. The structure and the catalytic properties for CO oxidation were investigated. CO catalytic oxidation was performed in the cylinders monolithic reactor (i.d., 8 mm; length, 50 mm) at atmospheric pressure. The mixture gas was 1 vol.% CO in air. The phase structure of the samples was characterized by X-ray powder diffraction (XRD) using a Rigaku D/Max 2500 VB2+/PC diffractometer with a Cu Ka radiation operating at 200 mA and 40 kV. Temperature programmed reduction (TPR) experiments were performed using a Thermo Electron Corporation TPD/R/O 1100 series catalytic surfaces analyzer equipped with a TCD detector. The samples were preheated with 10%O2/He mixture heating 20oC/min up to 500 oC, then cooling down to room temperature in flowing N2, and thereafter reduced with 5% H2/N2 mixture heating 20oC/min up to 1000oC. The catalytic activity, XRD and H2-TPR results were presented from Fig. 1 to Fig. 3. It was indicated that the CuxCe1-xO2-x/SBA-15/FeCrAl metallic monolithic catalysts have a good the catalytic oxidation activity for CO. 100 ▼ 359 ■ ■ ▼ 299 ▼ 60% 60 40 50% 10% 20% 30% 40% 50% 60% 20 40% ★ Intensity(a.u.) 60% Intensity(a.u.) CO conversion (%) 306 ▼ 80 264 50% 303 30% ● ● ● ● ★●● ● 368 40% 342 20% 10% ● 351 30% 20% 390 a 10% 459 0 50 100 150 200 250 300 350 400 450 500 550 600 Temperature (℃) Fig. 1. CO catalytic oxidation over the Cu0.5Ce0.5O1.5/SBA-15/ FeCrAlcatalysts GHSV = 36000 ml/g·h. 20 40 2 theta (deg) 60 80 Fig. 2. XRD patterns of the samples: (a) FeCrAl foil o pre-oxidized at 950 C for 15 h and x%Cu0.5Ce0.5O1.5/SBA-15/ FeCrAl (★) FeCr; (▼) Ce1-xCuxO2-x; (●) -Al2O3. (◆)CuO 200 400 600 Temperaturer/℃ 800 1000 Fig. 3. TPR patterns of the x%Cu0.5Ce0.5O1.5/SBA-15/FeCrAl samples