Uploaded by
chocolate candy
Chemistry Practice Worksheet: Atomic Structure & Electron Config
advertisement

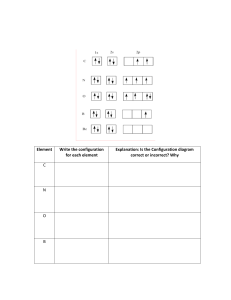
PRACTICE FOR DT 1-CHEMISTRY 1. Potassium has an atomic number of 19 and a mass number of 39. The number of electrons in the Potassium ion is 19 (because atoms have no charge so the number of electrons = number of protons) 2. Determine the protons, electrons and neutrons of the following atoms! a. 40 18𝑋 p = 18, e = 18, n = 40 – 18 = 22 b. 56 26𝐿 p = 26, e = 26, n = 56 – 26 = 30 c. 226 88𝑅 p = 88, e = 88, n = 226 – 88 = 138 2+ d. 52 24𝑌 p = 24, e = 22 (because it lose 2 electrons), n = 52 – 24 = 28 e. 210 85𝐾 p = 85, e = 86 (because it gain 1 electron), n = 210 – 85 = 125 3. Ion Z2- if it has atomic number 8 and mass number 18, what are the number of protons, electrons, and neutrons? p=8 e = 10 (because it gain 2 electron) n = 18 – 8 = 10 4. Determine the electron configuration of the following atoms! The outer shell of elements never have more than 8 electrons a. 21Sc 2, 8, 8, 1 b. 32Ge 2, 8, 18, 4 c. 54Xe 2, 8, 18, 18, 8 d. 15P 2, 8, 5 5. Determine the electron configuration of the following atoms (using spdf)! a. 33Q 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 b. 25X 1s2 2s2 2p6 3s2 3p6 4s2 3d5 c. 26Y3+ 2 2 6 2 6 1 5 26Y (neutral atoms) = 1s 2s 2p 3s 3p 4s 3d 3+ 2 2 6 2 6 3 26Y (lose 3 electrons) = 1s 2s 2p 3s 3p 3d d. 16Z22 2 6 2 4 16Z (neutral atoms) = 1s 2s 2p 3s 3p 22 2 6 2 6 16Z (gain 2 electrons) = 1s 2s 2p 3s 3p 6. Determine the location of atoms on the periodic table based on their electron configuration! a. 17J 1s2 2s2 2p6 3s2 3p5 p-block (group 17, period 3) b. 24R 1s2 2s2 2p6 3s2 3p6 4s2 3d4 d-block (group 6, period 4) c. 30W d. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 d-block (group 12, period 4) e. 5X 1s2 2s2 2p1 p-block (group 13, period 2) 7. Draw the orbitals of the following atoms! a. 25Y 1s2 2s2 2p6 3s2 3p6 4s2 3d5 ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿ ↿ ↿ ↿ ↿ ↿ ↿ ↿ b. 15T 2 1s 2s2 2p6 3s2 3p3 ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂