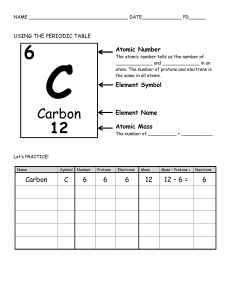
History of the Atom IMPORTANT PEOPLE 1) Democritus: o When you keep on dividing something in half, you get something really small. (Atomos) o First person to come up with the idea of atoms. o The world was made of tiny particles or “Atomos” which means indivisible— discontinuous theory o Smallest particles of matter cannot be destroyed 2) John Dalton: o Came up with modern atomic theory. o Dalton’s Model: (1807) Atom was indivisible, uniformly dense, solid sphere that participated in , but was unchanged by chemical rex Think of a marble hard and solid o Dalton’s Law of Multiple Proportions: The mass of one element forms a simple whole number ratio when combining with a fixed mass of another element Example: water H2O O = 16 g hydrogen peroxide H2O2 O = 32 g 16:32 = 1:2 3) JJ Thomson: o Discovered the proton (1885) and electron (1897) o Used a cathode ray. (proof of the existence of the electron) In a cathode ray tube, electrons travel from a negatively charged cathode to a positively charged anode. When he added the deflecting coils (magnets), he was able to deflect the beam of light Since the cathode ray was deflected toward the positive plate, it must be a composed of negatively charged particles Can see where hits because fluorescent screen glows Used in TV and computer screens o Plum pudding model (1903) electrons imbedded in a solid sphere of positive charge 4) Ernest Rutherford: o Gold-foil experiment. Atom is mostly empty space Has a small densely packed positively charged core Disperses the plum pudding model o Discovered the nucleus. o Rutherford Model 5) Niels Bohr: Disproved Rutherford’s Model. Bohr model (1913) Electrons in the atom can exist in stationary states which emit no radiation known as the planetary model electrons act like particles 6) Schrodinger: o Quantum mechanical model o also known as the electron cloud or wave model o “probability regions" of finding an electron o Electrons are not particles, but waves. 7) Chadwick: o Discovers the neutron. o In contrast with the helium nuclei (alpha particles) which are positively charged, and therefore repelled by the considerable electrical forces present in the nuclei of heavy atoms, this new tool in atomic disintegration need not overcome any electric barrier and is capable of penetrating and splitting the nuclei of even the heaviest elements 8) Werner Heisenberg: o Both Bohr and Schrodinger are correct. o Correct description of an atom. o Quantum theory o Electrons act like both particles and waves o Uncertainty principle: The position and the velocity of an object cannot both be measured exactly, at the same time, even in theory. 9) Mendeleev: o Came up with modern day periodic table. o Periodic Law: Placed elements in horizontal rows by atomic mass and in columns by chemical properties. Problem: atomic mass doesn’t increase regularly o Modern Periodic Law: (Mosley) The properties of elements repeat periodically when the elements are arranged in increasing orders by their atomic number. 10) Lavoisier (1770s) o The Law of Conservation of Matter The mass of the reactants before a chemical reaction is equal to the mass of the products after the reaction Matter (mass) can’t be created or destroyed, just rearrange o o •Democritus 400 B.C. •Proposes the idea of the atom 1770s •Lavoisier •Law of Conservation of Matter 1790s •Proust •Law of Definite Proportions 1807 •John Dalton •Founder of modern atomic theory (his model survived for almost 100 years) 1885 •J.J. Thomson •Discovery of the "proton" 1896 •Henri Becquerel •Discovery of Radioactivity 1897 •J.J. Thomson •Discovery of the "electron" 1903 •J.J. Thomson •"Plum-pudding model" 1909 •Ernest Rutherford •The Gold Foil Experiment 1911 •Ernest Rutherford •Rutherford's model of the atom 1911 •Robert Millikan •Oil drop experiment 1913 •Niels Bohr •Electrons exist in discrete energy levels 1923 •Robert Millikan •The Oil Drop Experiment- discovered the charge of an electron, discovered the mass p + = 1836 the mass of e- 1923 •Erwin Schrodinger •Wave Particle Duality 1925 •Erwin Schrodinger •Quantum Mechanical Model of the Atom 1932 •James Chadwick •Discovery of the "neutron" 1945 •Wolfgang Pauli •Pauli Exclusion Principle Atoms and Elements: Atomic Structure Atom The smallest unit of matter Has no charge, is electrically neutral Subatomic Particles Electron Symbol Relative Charge Relative Mass (amu) Actual Mass (g) Proton e-1 0 (1/1837) 9.11 x 10-28 Neutron p+ +1 1 n 0 1 1.673 x 10-24 1.675 x 10-24 Atomic # (Z) = The # of protons in a nucleus. The # of electrons. (ONLY in a neutral atom) Nucleon (protons/neutrons): Particles that occupy the nucleus including the proton and neutron Atomic Symbols Atomic number (Z) o o The number of protons in the nucleus **atoms of the same element must ALWAYS have the same number of protons Atomic Mass (A) The number of protons plus neutrons in the nucleus Also known as the mass number Expressed as atomic mass units or amu 1 amu = 1.66 x 10 -24 grams 1 amu = 1 proton = 1 neutron An electron has 1/1837th the mass of a proto Isotopes: Atoms of a single element (same atomic #) that differ in the # of neutrons in their nucleus (diff masses) amu = atomic mass units a very, very small unit of mass used to express the mass of atoms and molecules atomic mass unit is equal to one-twelfth of the mass of the nucleus of a carbon-12 atom. *If you change the # of protons, you are changing the element* Mass # (A) = The combined # of protons and neutrons in a nucleus A=Z+N PROTIUM DEUTERIUM 2 H 3 H 1 Another way to represent isotopes: [element name – hyphen] + + + Z1 - - - A1 TRITIUM Hyphen notation: Ex: Carbon – 13 H 1 the isotopes of Hydrogen Atoms, Ions and Isotope Ions: If you don’t have an equal number of protons and electrons, you have an ion Particles with an electric charge Atoms of the same element with different number of electrons Different net charge Number of protons ≠ number of electrons Cation: a positively charged ion Anion: a negatively charged ion Loss of electrons yields a positive charge Gain of electrons yields a negative charge Ex: C+(1) - 6 protons, 5 electrons B-(1) - 5 protons, 6 electrons When you have an imbalance between protons and electrons, it is no longer an atom, it is an ion A neutral atom would look like C - 6 protons, 6 electrons A cation is a positive ion. (+) An anion is a negative ion. (-) Average Atomic Mass Element: Phosphorus Ratio of Isotopes: 28 14 P 92 Equation 29 14 : P 30 14 5 : P 3 = (28 x 92) + (29 x 5) + (30 x 3) = 100 = (isotope abundance x isotope mass #) isotope abundance 28.11 Relative Abundance (of each isotope)