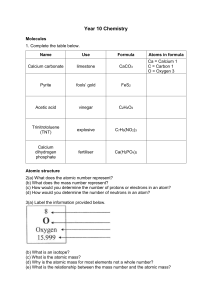
Explain how the atomic size increases as we move down the group. Annotate the position of electrons in the figure on the left. What do you understand by the term ‘shielding’ Explain why the size of an atom gets smaller as you move from the left side of the periodic table to the right side? Add in the electrons in the below mentioned structures. What is electronegativity? Is there a relation to the atomic radii graph from the question before?