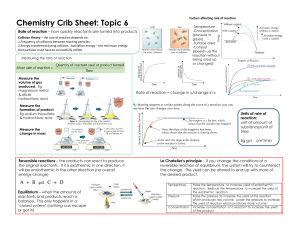
Topic 6 MC [24 marks] 1. [1 mark] Which instrument would best monitor the rate of this reaction? 2KI (aq) + Cl2 (aq) → 2KCl (aq) + I2 (aq) A. Balance B. Colorimeter C. Volumetric flask D. Gas syringe 2. [1 mark] Which combination has the greatest rate of reaction at room temperature? 3. [1 mark] On the following Maxwell-Boltzmann distribution, which letter represents activation energy? A. A B. B C. C D. D 4. [1 mark] Which change causes the greatest increase in the initial rate of reaction between nitric acid and magnesium? 2HNO3 (aq) + Mg (s) → Mg(NO3)2 (aq) + H2 (g) 5. [1 mark] The graph shows the Maxwell–Boltzmann energy distribution curve for a given gas at a certain temperature. How would the curve change if the temperature of the gas decreases while the other conditions remain constant? A. The maximum would be lower and to the left of M. B. The maximum would be lower and to the right of M. C. The maximum would be higher and to the left of M. D. The maximum would be higher and to the right of M. 6. [1 mark] Which arrow shows the activation energy of the uncatalysed forward reaction for this equilibrium? 2𝑆𝑂2 (𝑔) + 𝑂2 (𝑔) ⇌ 2𝑆𝑂3 (𝑔) 7. [1 mark] 𝐻 = −196 𝑘𝐽 𝑚𝑜𝑙 −1 The dotted line represents the volume of carbon dioxide evolved when excess calcium carbonate is added to hydrochloric acid. Which graph represents the production of carbon dioxide when excess calcium carbonate is added to the same volume of hydrochloric acid of double concentration? 8. [1 mark] Which properties can be monitored to determine the rate of the reaction? Fe (s) + CuSO4 (aq) → Cu (s) + FeSO4 (aq) I. change in volume II. change in temperature III. change in colour A. I and II only B. I and III only C. II and III only D. I, II and III 9. [1 mark] Which will increase the rate of reaction between calcium carbonate and hydrochloric acid? I. an increase in temperature II. an increase in concentration of hydrochloric acid III. an increase in particle size of calcium carbonate A. I and II only B. I and III only C. II and III only D. I, II and III 10. [1 mark] The dotted line represents the formation of oxygen, O2(g), from the uncatalysed complete decomposition of hydrogen peroxide, H2O2 (aq). Which curve represents a catalysed reaction under the same conditions? 11. [1 mark] The same amount of two gases, X and Y, are in two identical containers at the same temperature. What is the difference between the gases? A. X has the higher molar mass. B. Y has the higher molar mass. C. X has the higher average kinetic energy. D. Y has the higher average kinetic energy. 12. [1 mark] Samples of sodium carbonate powder were reacted with separate samples of excess hydrochloric acid. Na2CO3 (s) + 2HCl (aq) → CO2 (g) + 2NaCl (aq) + H2O (l) Reaction I: 1.0 g Na2CO3 (s) added to 0.50 mol dm−3 HCl (aq) Reaction II: 1.0 g Na2CO3 (s) added to 2.0 mol dm−3 HCl (aq) What is the same for reactions I and II? A. Initial rate of reaction B. Total mass of CO2 produced C. Total reaction time D. Average rate of production of CO2 13. [1 mark] What decreases the activation energy of a reaction? A. Increasing the temperature B. Adding a catalyst C. Adding more reactants D. Increasing collision frequency of reactants 14. [1 mark] Which change increases the rate of formation of hydrogen when zinc reacts with excess hydrochloric acid, assuming all other conditions remain the same? Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) A. Adding water to the hydrochloric acid B. Decreasing the temperature C. Increasing the volume of hydrochloric acid D. Decreasing the size of the zinc particles while keeping the total mass of zinc the same 15. [1 mark] Which statements are correct? I. The activation energy of a reaction is not affected by temperature. II. A catalyst reduces the enthalpy change of a reaction. III. Catalysts provide alternative reaction pathways. A. I and II only B. I and III only C. II and III only D. I, II and III 16. [1 mark] Which factors can affect the rate of reaction? I. Particle size of solid reactant II. Concentration of reacting solution III. Pressure of reacting gas A. I and II only B. I and III only C. II and III only D. I, II and III 17. [1 mark] The diagram shows the energy profile for a catalysed and uncatalysed reaction. Which represents the enthalpy change, ΔH, and the activation energy, Ea, for the catalysed reaction? 18. [1 mark] Excess magnesium powder was added to a beaker containing hydrochloric acid, HCl (aq). The mass of the beaker and its contents was recorded and plotted against time (line I). Which change could give line II? A. Doubling the mass of powdered Mg B. Using the same mass of Mg ribbon C. Increasing the temperature D. Using the same volume of more concentrated HCl 19. [1 mark] 100 cm3 of 10% hydrogen peroxide solution decomposes at 298 K to form water and oxygen. 1 H2O2(aq) → H2O(l) + 2O2(g) The dotted line graph represents the volume of oxygen produced. Which graph represents the decomposition of an equal volume of a 20% solution under the same conditions? 20. [1 mark] CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g) Which change does not increase the initial rate of reaction when CaCO3(s) is added to excess HCl(aq)? A. Decrease in the size of the CaCO3(s) particles B. Increase in the temperature of the reaction mixture C. Increase in the concentration of HCl(aq), keeping the same volume D. Increase in the volume of HCl(aq), keeping the same concentration 21. [1 mark] Which conditions must be met for a reaction to take place? I. Reactants collide with sufficient energy. II. Reactants collide with correct orientation. III. Reactants must be in the same state. A. B. C. D. I and II only I and III only II and III only I, II and III 22. [1 mark] For the reaction R → P, which letter represents the activation energy for the catalysed reverse reaction? 23. [1 mark] Graph 1 shows a plot of volume of CO2(g) against time for the reaction of CaCO3(s) with 1.00 moldm−3HCl (aq). The acid is the limiting reagent and entirely covers the lumps of CaCO3(s). Which set of conditions is most likely to give the data plotted in graph 2 when the same mass of CaCO3(s) is reacted with the same volume of HCl(aq) at the same temperature? 24. [1 mark] Which experimental methods could be used to observe the progress of the following reaction? Cr2O72-(aq) + 6I-(aq) + 14H+(aq) → 2Cr3+(aq) + 3I2(aq) + 7H2O(l) I. Change in colour II. Change in mass III. Change in electrical conductivity A. I and II only B. I and III only C. II and III only D. I, II and III Printed for AMER SCH OF MILAN-HS © International Baccalaureate Organization 2022 International Baccalaureate® - Baccalauréat International® - Bachillerato Internacional®