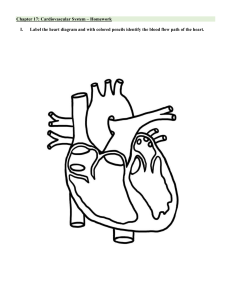
Cardiac System Notes Dysrhythmias Properties of Cardiac Cells Term Definition/Meaning/Significance Automaticity Ability to initiate an impulse spontaneously and continuously Contractility Ability to respond mechanically to an impulse Conductivity Ability to transmit an impulse along a membrane in an orderly manner Excitability Ability to be electrically stimulated Nervous System Control of Heart Function Autonomic Rate of impulse formation Speed of conduction Strength of contraction Parasympathetic Decreases rate Slows impulse conduction Decreases force of contraction Sympathetic Increases rate Increases force of contraction Electrical activity P wave PR segment QRS complex ST segment T wave QT Isoelectric line Represents atrial depolarization time taken for impulse to spread through atria ventricular depolarization end of depolarization and beginning of repolarization of ventricles ventricular repolarization time taken for entire electrical depolarization and repolarization of ventricles baseline Systematic approach to ECG interpretation Step How to Regularity • Is the rhythm regular? • Print strip and measure R - R • Is the rhythm irregular? • Are there patterns to the irregularity? • Any ectopic beats? Early? Late? Rate • Count • • • • the number of QRS complexes in 1 minute the R-R intervals in 6 seconds, and multiply by 10 number of small squares between one R-R interval, and divide this number by 1 500 number of large squares between one R-R interval, and divide this number by 300 Number of R-R intervals = 8 8 x 10 = 80 bpm P waves • • • Are they present? Upright One for every QRS Large squares between R-R interval = ~3 = 300/3 = 100 bpm Small squares between R-R interval = ~17 = 1500/17 = 88 bpm • Precedes the QRS PR interval • • • Are they constant? Normal measure 0.12-0.20 seconds Short? Long? QRS complex • • • Normal measure – 0.06-0.10 sec Are they all the same configuration? Any ectopic beats? • Supraventricular – impulse begins above the AV node/ventricles (usually narrow QRS) • Ventricular – impulse began below the AV node (wide QRS) • BBB – bundle branch block Rhythm Normal Sinus Rhythm Rate 60-100 bpm P Wave Upright in Lead II; one per QRS: Uniform shape PR Interval 0.12-0.20 sec; constant QRS Complex 0.06-0.10 sec <12 Sinus Bradycardia <60 bpm Upright in Lead II; one per QRS: Uniform shape 0.12-0.20 sec; constant normal shape and duration <12 Sinus Tachycardia 101-160 bpm Upright in Lead II; one per QRS: Uniform shape 0.12-0.20 sec; constant normal shape and duration <12 Premature Atrial Contraction (PAC) Can occur at any rate unable to be measured <12; QRS absent after nonconducted PAC Paroxysmal Supraventricular Tachycardia 161-250 bpm, once in atrial tach Shaped differently from sinus Ps; often hidden in preceding T wave Shaped differently from sinus Ps but same as each other unable to be measured <12 Atrial Flutter 251-350 bpm None; flutter unable to be waves present measured (zigzag or sawtooth waves) normal shape and duration <12 Example Atrial Fibrillation 350-700 bpm None; fibrillatory waves present (waviness of the baseline) unable to be measured normal shape and duration <12 Premature Ventricular Complex (PVC) Can occur at any rate Usually none unable to be measured <12; wide and bizarre in shape Ventricular Tachycardia 100-250 bpm Dissociated if even present unable to be measured <12; wide and bizarre in shape Ventricular Fibrillation Cannot be counted None unable to be measured None; just a wav baseline that looks like static Asystole Zero None None none Rhythm Sinus Bradycardia Clinical Associations Clinical Manifestations • Occurs in response to • Hypotension • Carotid sinus • Pale, cool skin massage • Weakness • Valsalva’s maneuver • Angina • Hypothermia • Dizziness or syncope • Increased intraocular • Confusion or pressure disorientation • Increased vagal tone • Shortness of breath • Administration of parasympathomimeti c drugs • Occurs in disease states • Hypothyroidism • Increased intracranial pressure • Obstructive jaundice • Inferior wall MI Sinus Tachycardia Associated with physiological and psychological stressors: • Exercise • Fever • Dizziness, dyspnea, and hypotension due to decreased CO Treatment • Atropine • Pacemaker may be required Determined by underlying cause • -adrenergic blockers to reduce HR and myocardial oxygen consumption Premature Atrial Contraction (PAC) Paroxysmal Supraventricular Tachycardia • • • • • • • • • • • • • • • • Pain Hypotension Hypovolemia Anemia Hypoxia Hypoglycemia MI Heart failure Hyperthyroidism Anxiety, fear Can result from • Emotional stress • Physical fatigue • Use of caffeine, tobacco, alcohol • Hypoxia • Electrolyte imbalances • Hyperthyroidism • COPD • Heart disease including CAD and valvular disease In a normal heart • Overexertion, deep inspiration • Emotional stress • Stimulants Digitalis toxicity Rheumatic heart disease CAD Cor pulmonale • Increased myocardial oxygen consumption may lead to angina • Isolated PACs are not significant in those with healthy hearts In persons with heart disease, may be warning of more serious dysrhythmia • • • • • Prolonged episode and HR >180 bpm may precipitate decreased CO Hypotension Dyspnea Angina • • Antipyretics to treat fever Analgesics to treat pain Depends on symptoms • -adrenergic blockers may be used to decrease PACs • Reduce or eliminate caffeine • • • Vagal maneuvers: Valsalva, coughing IV adenosine If vagal maneuvers and/or drug therapy is ineffective and/or client becomes hemodynamically unstable, DC cardioversion should be used Atrial Flutter Atrial Fibrillation • Usually occurs with • CAD • Hypertension • Mitral valve disorders • Pulmonary embolus • Chronic lung disease • Cor pulmonale • Cardiomyopathy • Hyperthyroidism • Drugs: digoxin, quinidine, epinephrine • Usually occurs with underlying heart disease: • Rheumatic heart disease • CAD • Cardiomyopathy • Hypertensive heart disease • HF • Pericarditis Often acutely caused by: • Thyrotoxicosis • Alcohol intoxication • Caffeine use • Electrolyte disturbances • • • • • High ventricular rates (>100) and loss of the atrial “kick” can decrease CO and precipitate HF, angina Risk for stroke due to risk of thrombus formation in the atria Can result in decrease in CO due to ineffective atrial contractions (loss of atrial kick) and rapid ventricular response Thrombi may form in the atria as a result of blood stasis Embolus may develop and travel to the brain, causing a stroke Primary goal is to slow ventricular response by increasing AV block • Drugs to slow HR: calcium channel blockers, adrenergic blockers • Electrical cardioversion may be used to convert the atrial flutter to sinus rhythm emergently and electively • Antidysrhythmic drugs (e.g., amiodarone, propafenone) to convert atrial flutter to sinus rhythm or to maintain sinus rhythm • Radiofrequency catheter ablation can be curative therapy for atrial flutter • Goals: • Decrease ventricular rate. • Prevent cerebral embolic events • Drugs for rate control: calcium channel blockers (e.g., diltiazem) and βadrenergic blockers (e.g., metoprolol) • Long-term anticoagulation therapy • For some clients, conversion to sinus rhythm may be considered • • Premature Ventricular Complex (PVC) • • • • • • Stress Cardiac surgery Stimulants: caffeine, alcohol, nicotine, aminophylline, epinephrine, isoproterenol and digoxin Electrolyte imbalances Hypoxia Fever Disease states: MI, mitral valve prolapse, HF, CAD • • • Ventricular Tachycardia • • • • • • MI CAD Electrolyte imbalances Cardiomyopathy Mitral valve prolapses Long QT syndrome • • In normal heart, usually benign In heart disease, PVCs may decrease CO and precipitate angina and HF if >10/minute • Monitor client’s response to PVCs • PVCs often do not generate a sufficient ventricular contraction to result in a peripheral pulse Represents ventricular irritability VT can be stable (client has a pulse) or unstable (client is pulseless) Sustained VT: severe decrease in CO • Hypotension • • • Antidysrhythmic drugs used for conversion: amiodarone, propafenone • The nurse must determine that the client has had atrial fibrillation or atrial flutter for less than 48 hours Based on cause of PVCs • Oxygen therapy for hypoxia • Electrolyte replacement • Other treatment according to symptoms Precipitating causes must be identified and treated (e.g., electrolyte imbalances, ischemia) Monomorphic VT • • • • Digitalis toxicity Central nervous system disorders • • • Pulmonary edema Decreased cerebral blood flow • Cardiopulmonary arrest Treatment for VT must be rapid May recur if prophylactic treatment is not initiated Ventricular fibrillation may develop • • • • Hemodynamically stable (present pulse) + preserved LV function: IV amiodarone • Hemodynamically unstable or poor LV function: IV amiodarone followed by cardioversion Polymorphic VT with a normal baseline QT interval: -adrenergic blockers, amiodarone, or sotalol • Cardioversion is used if drug therapy is ineffective Polymorphic VT with a prolonged baseline QT interval: IV magnesium and antitachycardia pacing • Drugs that prolong the QT interval should be discontinued • If the rhythm is not converted, cardioversion may be needed VT without a pulse is a lifethreatening situation • Cardiopulmonary resuscitation (CPR) and rapid defibrillation • Ventricular Fibrillation • • • Asystole • • • • • • • • Pulseless Electrical Activity (PEA) • • • • • Acute MI, CAD, cardiomyopathy May occur during cardiac pacing or cardiac catheterization May occur with coronary reperfusion after fibrinolytic therapy Accidental electric shock Hyperkalemia Hypoxia Acidosis Drug toxicity Advanced cardiac disease Severe cardiac conduction system disturbance End-stage HF • Electrical activity can be observed on the ECG, but no mechanical activity of the ventricles is evident, and the client has no pulse Prognosis is poor unless the underlying cause is identified and quickly corrected Hypovolemia Hypoxia Metabolic acidosis • • • • Unresponsive, pulseless, and apneic state If not treated rapidly, death will result • Unresponsive, pulseless, and apneic state Prognosis for asystole is extremely poor • Unresponsive, pulseless, and apneic state • • • • Epinephrine if defibrillation is unsuccessful Assessment of circulation, airway, and breathing (CAB) Immediate initiation of CPR and advanced cardiac life support (ACLS) measures with the use of defibrillation and definitive drug therapy CPR with initiation of ACLS measures (e.g., intubation, transcutaneous pacing, IV therapy with epinephrine and atropine) CPR followed by intubation and IV epinephrine Epinephrine 1 mg every 3–5 minutes Treatment is directed toward correction of the underlying cause • Hyperkalemia or hypokalemia Hypothermia Drug overdose Cardiac tamponade MI Tension pneumothorax Pulmonary embolus • • • • • • Heart Failure Terms • Pathophysiology • • • • • • Cardiac output – measurement of blood pumped by each ventricle in 1 minute • CO = SV x HR Stroke volume – amount of blood ejected from the ventricle with the heartbeat Preload – volume of blood in the ventricles at the end of diastole, before the next contraction Afterload – peripheral resistance against which the LV must pump When heart cannot maintain pumping capability • Cardiac output or stroke volume decreases • Less blood reaches the various organs • Decreased cell function • Fatigue and lethargy • Mild acidosis develops • Backup and congestion develop as coronary demands for oxygen and glucose are not met • Output from ventricle is less than the inflow of blood • Congestion in venous circulation draining into the affected side of the heart Heart failure with preserved ejection fraction • Impaired ability of the ventricles to relax and fill during diastole, resulting in decreased stroke volume and CO • Diagnosis based on the presence of heart failure symptoms and normal EF Mixed heart failure • Seen in disease states such as dilated cardiomyopathy (DCM) • Poor EFs (<35%) • High pulmonary pressures • • • Biventricular failure • Both ventricles may be dilated and have poor filling and emptying capacity Compensatory mechanisms are activated to maintain adequate CO • Sympathetic nervous system (SNS) activation: first and least effective mechanism • Release of catecholamines (epinephrine and norepinephrine) • Increased heart rate (HR) • Increased myocardial contractility • Peripheral vasoconstriction • Initially, increased HR and contractility improve CO • Over time, these mechanisms are detrimental as they increase the workload of the failing myocardium and the need for O2 • Neurohormonal responses: Kidneys release renin • Renin converts angiotensinogen to angiotensin I • Angiotensin I is converted to angiotensin II by a converting enzyme made in the lungs • Angiotensin II causes • Adrenal cortex to release aldosterone (sodium and water retention) • Increased peripheral vasoconstriction (increases BP) • Response is known as the renin–angiotensin–aldosterone system (RAAS) • Neurohormonal responses • Low CO causes a decrease in cerebral perfusion pressure • Antidiuretic hormone (ADH) is secreted and causes • increased water reabsorption in the renal tubules, leading to water retention and increased blood volume • Endothelin is stimulated by ADH, catecholamines, and angiotensin II, causing • arterial vasoconstriction • increase in cardiac contractility • Hypertrophy • Proinflammatory cytokines (e.g., tumour necrosis factor): released by cardiac myocytes in response to cardiac injury • Depression of cardiac function by causing cardiac hypertrophy, contractile dysfunction, and death of myocytes Consequences of compensatory mechanisms • Dilation • Etiology Enlargement of the chambers of the heart that occurs when pressure in the left ventricle is elevated • Initially an adaptive mechanism • Eventually this mechanism becomes inadequate, and CO decreases • Hypertrophy • Increase in muscle mass and cardiac wall thickness in response to chronic dilation, resulting in • Poor contractility • Higher O2 needs • Poor coronary artery circulation • Risk for ventricular dysrhythmias • Counterregulatory processes • Natriuretic peptides: atrial natriuretic peptide (ANP), b-type natriuretic peptide (BNP) • Released in response to increase in atrial volume and ventricular pressure • Promote venous and arterial vasodilation, reducing preload and afterload • Chronic HF leads to a depletion of these factors • Heart failure with reduced ejection fraction (most common) • Caused by: • Impaired contractile function (e.g., MI) • Increased afterload (e.g., hypertension) • Cardiomyopathy • Mechanical abnormalities (e.g., valve disease) • Heart failure with preserved ejection fraction • Caused by • Left ventricular hypertrophy from chronic hypertension • Aortic stenosis • Hypertrophic cardiomyopathy Primary Causes Precipitating Causes Types of Heart Failure Clinical Manifestations • Left-sided HF (most common) from left ventricular dysfunction (e.g., MI hypertension, CAD, cardiomyopathy) • Backup of blood into the left atrium and pulmonary veins causes pulmonary congestion • Dyspnea and orthopnea • Develops as fluid accumulates in the lungs • Cough • Associated with fluid irritating the respiratory passages • Paroxysmal nocturnal dyspnea • Indicates the presence of acute pulmonary edema • Usually develops during sleep • Excess fluid in lungs frequently leads to infections such as pneumonia • Right-sided HF from left-sided HF, cor pulmonale, right ventricular MI • Backup of blood into the right atrium and venous systemic circulation • Jugular venous distension • Hepatomegaly, splenomegaly • Vascular congestion of GI tract • Peripheral edema Acute Decompensated Heart Failure (ADHF) • Pulmonary edema, often life-threatening • Early • Increase in the respiratory rate Diagnostic Studies • Decrease in PaO2 • Later • Tachypnea • Respiratory acidemia • Physical findings • Orthopnea • Dyspnea, tachypnea • Use of accessory muscles • Cyanosis • Cool and clammy skin • Cough with frothy, blood-tinged sputum • Breath sounds: crackles, wheezes, rhonchi • Tachycardia • Hypotension or hypertension Chronic HF • Fatigue • Dyspnea, orthopnea, paroxysmal nocturnal dyspnea • Persistent, dry cough, unrelieved with position change or over-the-counter cough suppressants • Tachycardia • Dependent edema • Edema may be pitting in nature • Sudden weight gain of >2 kg (4 lb) in 2 days may indicate an exacerbation of HF • Nocturia • Skin • Dusky, cool, damp to touch • Lower extremities: shiny and swollen, diminished or absent hair growth, pigment changes • Restlessness, confusion, decreased memory • Chest pain (angina) • Weight changes • Anorexia, nausea • Fluid retention • Primary goal: Determine and treat underlying cause • History and physical examination Nursing and Collaborative Management • • Chest x-ray • ECG • Lab studies (e.g., cardiac enzymes, BNP) • Hemodynamic assessment • Echocardiogram • Stress testing • Cardiac catheterization • Ejection fraction Overall goals of therapy for ADHF and chronic HF • Decrease client symptoms • Improve LV function • Reverse ventricular remodeling • Improve quality of life • Decrease mortality and morbidity ADHF • Decrease intravascular volume • Reduces venous return and preload • Loop diuretics (e.g., furosemide) • Ultrafiltration or aquapheresis • Decrease venous return (preload) • Reduces the amount of volume returned to the LV during diastole • High Fowler’s position • IV nitroglycerin • Decrease afterload • Improves CO and decreases pulmonary congestion • Careful monitoring of vital signs is crucial • Improve gas exchange and oxygenation • Supplemental oxygen • Morphine sulphate • Noninvasive ventilatory support (BiPAP) • Improve cardiac function • For clients who do not respond to conventional pharmacotherapy (e.g., diuretics, vasodilators, morphine sulphate) • Inotropic therapy • Dobutamine, milrinone • Hemodynamic monitoring • Reduce anxiety • Distraction, imagery • Sedative medications (e.g., morphine sulphate) Chronic HF • Main treatment goals • Treat the underlying cause and contributing factors • Maximize CO • Provide treatment to alleviate symptoms • Improve ventricular function • Improve quality of life • Preserve target organ function • Improve mortality and morbidity • Oxygen administration • Self-management teaching • Exercise and activity • Devices • Cardiac resynchronization therapy (CRT) or biventricular pacing • Implantable cardioverter defibrillator • Nonpharmacological therapies • Mechanical circulatory support • Intra-aortic balloon pump (IABP) • Extra-corporeal Membrane Oxygenation (ECMO) • Ventricular assist device (VAD) • Therapeutic goals for drug therapy • Identification of type of HF and causes • Correction of sodium and water retention and volume overload • Reduction of cardiac workload • Improvement of myocardial contractility • Control of precipitating and complicating factors • Drug therapy • • • • • Diuretics • Thiazide • Loop • ACE inhibitors • Neprilysin inhibitors • -Adrenergic blockers • Positive inotropic agents • Dobutamine and milrinone are the two most commonly used agents Nutritional therapy • Diet education and weight management: Individualize recommendations and consider cultural background • Recommend Dietary Approaches to Stop Hypertension (DASH) diet • Sodium is usually restricted to 2 g per day • Fluid restriction not generally required • Daily weights are important • Same time, same clothing each day • Weight gain of 2 kg (4 lb) over 2 days or a 2.5 kg (5-lb) gain over a week should be reported to health care provider Assessment • Subjective data • Past health history • Functional health patterns • Medications • Objective data • Physical examination Nursing diagnoses • Decreased cardiac output • Excess fluid volume • Impaired gas exchange • Activity intolerance Planning: Overall goals • Decrease in symptoms (e.g., shortness of breath, fatigue) • Decrease in peripheral edema • • • Complications • • • • • • Increase in exercise tolerance • Adherence to drug regimen • No complications related to HF Implementation: Client education • Medications (lifelong) • Taking pulse rate • Know when drugs (e.g., digitalis, -adrenergic blockers) should be withheld and reported to health care provider Implementation: Client and caregiver education • Home BP monitoring • Signs of hypokalemia and hyperkalemia if taking diuretics that deplete or spare potassium • Instruct client in energy-conserving and energy-efficient behaviours Evaluation • Respiratory status • Fluid balance • Activity tolerance • Anxiety control • Knowledge of disease process Pleural effusion Dysrhythmias • Promotes thrombus/embolus formation, increasing risk for stroke • Treatment can include rate control, cardioversion, antidysrhythmic, and/or systemic anticoagulation Left ventricular thrombus HF can lead to severe hepatomegaly Renal insufficiency or failure Obstructive pulmonary disease Description Asthma • Asthma is a chronic inflammatory lung disorder of the airways that COPD • Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease state characterized by airflow limitation that is not fully reversible • Etiology • results in recurrent episodes of airflow obstruction, but is usually reversible Inflammation causes varying degrees of obstruction in the airways, which leads to recurrent episodes of: • Wheezing • Breathlessness • Sensation of chest tightness • Cough, particularly at night and in the early morning • Although the exact mechanisms that cause airway hyper-responsiveness and inflammation remain unknown, multiple triggers are involved: • Allergens • Exercise • Respiratory infections • Nose and sinus problems • Drugs and food additives • Cold, dry air • Stress • Hormones, menses • GERD • Occupational exposure • • • The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases, primarily caused by cigarette smoking Airflow limitation not fully reversible • Usually progressive • Abnormal inflammatory response of airway and lungs to noxious particles or gases COPD patients display characteristics of • Chronic bronchitis • Emphysema Risk factors • Cigarette smoking • Occupational chemicals and dust • Air pollution • Infection • Heredity • Aging Cigarette Smoking • Clinically significant airway obstruction develops in 15% to 20% of smokers. • 80% to 90% of COPD cases related to tobacco smoking. • Effects of nicotine • Stimulates sympathetic nervous system • Increases HR • Causes peripheral vasoconstriction • Increases BP and cardiac workload • Compounds problems in CAD • Effects on respiratory tract • Increased production of mucus • Hyperplasia of goblet cells • Lost or decreased ciliary activity • Carbon monoxide • ↓ O2 carrying capacity • ↑ Heart rate • Impaired psychomotor performance and judgement • Passive smoking (second-hand smoke) • ↓ Pulmonary function • ↑ Risk of lung cancer • ↑ Rates of mortality from ischemic heart disease Environmental • COPD can develop with intense or prolonged exposure to • Dusts, vapours, irritants, or fumes. • High levels of air pollution. Infection • Recurring infections impair normal defense mechanisms. • Risk factor for COPD • Intensify pathological destruction of lung tissue • HIV infection • TB is a risk factor Hereditary • -Antitrypsin (AAT) deficiency • Genetic risk factor for COPD • Severe AAT deficiency occurs in 1 in 5000–1 in 5500 of Canadian and North American population. Aging • Some degree of the same symptoms of emphysema is common because of physiological changes of aging lung tissue. • Aging results in changes in the lung structure and respiratory muscles that cause a gradual loss of the • Pathophysiology • • • • • The hallmarks of asthma are airway inflammation and airway hyperresponsiveness. The degree of bronchoconstriction is related to the degrees of airway inflammation, airway hyperresponsiveness, and exposure to endogenous and exogenous triggers (e.g., infections, allergens, histamine, and other cell mediators). Exposure to allergens or irritants initiates an inflammatory cascade involving multiple cell types, mediators, and chemokines. Typically, there are two possible types of asthmatic responses to stimuli: an early-phase response and a late-phase response. Early-phase response is characterized by bronchospasm. • Increased mucus secretion, edema formation, and increased amounts of tenacious sputum cause wheeze, cough, sensation of chest tightness, shortness of breath or a combination • Peaks in 30–60 minutes after trigger exposure • • • • elastic recoil of the lung – as a result, the lungs become smaller and stiffer The number of functional alveoli decreases as a result of the loss of the alveolar supporting structures Defining features • Irreversible airflow limitations during forced exhalation due to loss of elastic recoil • Airflow obstruction due to mucus hypersecretion, mucosal edema, and bronchospasm Primary process is inflammation. • Inhalation of noxious particles • Mediators released cause damage to lung tissue • Airways inflamed • Parenchyma destroyed Supporting structures of lungs are destroyed. • Air goes in easily but remains in the lungs. • Bronchioles tend to collapse. • Causes barrel-chest look. Common characteristics • Mucous hypersecretion • Dysfunction of cilia • Hyperinflation of lungs • Gas exchange abnormalities • • Clinical manifestations • • • Subsides in about 30–90 minutes Late-phase response can be more severe than the early-phase response and is primarily inflammation. • Peaks in 5–12 hours • May last several hours to days • Corticosteroids are effective in preventing and reversing this cycle. • If airway inflammation is not treated or does not resolve, it may lead to irreversible lung damage. Unpredictable and variable • Recurrent episodes of wheezing, breathlessness, cough, and tight chest • Particularly at night or early morning (0200–0500 hours) • May be abrupt or gradual • Lasts minutes to hours Expiration may be prolonged. • Inspiration-expiration ratio of 1:2 prolonged to 1:3 or 1:4 • Bronchospasm, edema, and mucus in bronchioles narrow the airways • Air takes longer to move out Wheezing is unreliable to gauge severity. • • • • • • • • Develops slowly Diagnosis is considered with • Cough. • Sputum production. • Dyspnea. • Exposure to risk factors. Dyspnea usually prompts medical attention. • Occurs with exertion in early stages • Present at rest with advanced disease Causes chest breathing • Use of accessory and intercostal muscles • Inefficient Characteristically underweight with adequate caloric intake Anorexia Chronic fatigue Physical examination findings • Prolonged expiratory phase • • • • Complications • Severe attacks may have no audible wheezing. • Usually begins upon exhalation • “Silent chest” Cough variant asthma • Cough is the only symptom. • Bronchospasm is not severe enough to cause airflow obstruction. Difficulty with air movement • Client may feel increasingly anxious An acute attack usually reveals signs of hypoxemia. • Restlessness • ↑ anxiety • Inappropriate behaviour • ↑ pulse and blood pressure • Pulsus paradoxus (drop in systolic BP during inspiratory cycle >10 mm Hg) • Respiratory rate > 30 breaths/minute • Difficulty speaking in full sentences • Percussion reveals hyperresonance Severe acute attack • Common causes of severe acute attacks include viral illnesses, ingestion of Aspirin • • Wheezes • Decreased breath sounds • ↑ Anterior–posterior diameter Bluish-red colour of skin • Polycythemia and cyanosis Cor pulmonale • Hypertrophy of right side of heart • Result of pulmonary hypertension • • • or other NSAIDs, increases in environmental pollutants or other allergen exposure, and discontinuation of drug therapy. Clinical manifestations are similar to those of non-severe asthma but are more serious and prolonged. Complications may include pneumothorax, pneumomediastinum, acute cor pulmonale with right ventricular failure, and severe respiratory muscle fatigue that leads to respiratory arrest Respiratory arrest can be fatal. • Late manifestation of chronic pulmonary heart disease • Eventually causes right-sided heart failure • Dyspnea • Distended neck veins • Hepatomegaly with right upper quadrant tenderness • Peripheral edema • Weight gain • Ascites • Epigastric distress Exacerbations of COPD • Sustained worsening of symptoms • Signaled by change in usual • Dyspnea • Cough • Sputum • Associated with poorer outcomes • Primary causes • Infection • Noninfectious causes: Air pollution, allergens, irritants, cold air Acute respiratory failure • Caused by • Exacerbations • Cor pulmonale • Discontinuing bronchodilator or corticosteroid medication • Indiscriminate use of sedatives and opioids • Surgery or severe, painful illness involving chest or abdomen Peptic ulcer disease Depression/anxiety • • Diagnostic studies • • • • • • • • • Detailed history and physical exam Pulmonary function tests Peak flow monitoring Chest x-ray ABGs Oximetry Allergy testing Blood levels of eosinophils Sputum culture and sensitivity • • • • Collaborative care • • • • • • • Establishment of a confirmed diagnosis through the use of objective measures Development of a partnership between health care providers and the patients and families affected by asthma Limited exposure to triggers Education of patients Appropriate pharmacotherapy Continuous assessment and monitoring of asthma control and severity Implementation of a written action plan • • • • Approximately 40% of COPD clients experience depression. If client becomes anxious because of dyspnea, teach pursed-lip breathing. Diagnosis confirmed by pulmonary function tests • Chest x-rays, spirometry, history, and physical examination are also important in the diagnostic workup. Spirometry typical findings • Reduced FEV1/FVC ratio • Increased residual volume ABG typical findings • Low PaO2 • ↑ PaCO2 • ↓ pH • ↑ Bicarbonate level found in late stages of COPD Walk test (6 min.) to determine O2 desaturation in the blood with exercise ECG can show signs of right ventricular failure. Primary goals of care • Prevent progression. • Reduce frequency and severity of exacerbations. • Alleviate breathlessness. • Improve exercise tolerance and daily activity. • Treat exacerbations and complications. • Improve quality of life and reduce mortality risk. Smoking cessation • Most effective intervention • Accelerated decline in pulmonary function slows and usually improves. Drug therapy • Bronchodilators • Relax smooth muscle in the airway • • • • • • • Ensuring regular follow-up. Medications are divided into two general classifications: • Relievers (“rescue” medications used intermittently as required to ease asthma symptoms) • Controllers (maintenance therapy used on a daily basis, typically twice a day). Teach proper technique Use metered-dose inhaler (MDI) with spacer to facilitate uptake of medication Acute asthma episode • Often come to ED • Respiratory distress • Treatment depends upon severity and response to therapy. • Severity measured with flow rates • Oral corticosteroids • Therapy may be started and monitored with pulse oximetry or ABGs in severe cases Most therapeutic measures are the same as for acute episode. • ↑ in frequency and dose of bronchodilators • May require mechanical ventilation Severe attack • • • • • • Reduces airway resistance and dynamic hyperinflation of the lungs • Improve ventilation of the lungs • ↓ Dyspnea and ↑ FEV1 • Inhaled route is preferred. • Commonly used bronchodilators • β2-Adrenergic agonists • Anticholinergics • Methylxanthines • Long-acting anticholinergic • Tiotropium (Spiriva) • Inhaled corticosteroid therapy • Used for moderate to severe cases O2 therapy is used to • reduce work of breathing. • maintain PaO2. • reduce workload on the heart. Long-term O2 therapy improves • survival. • exercise capacity. • cognitive performance. • sleep in hypoxemic clients. O2 delivery systems are high or low flow. • Low flow is most common. • Low flow is mixed with room air, and delivery is less precise than high flow. Humidification • Used because O2 has a drying effect on the mucosa • Supplied by nebulizers, vapotherm, and bubblethrough humidifiers Complications of oxygen therapy • Combustion • • • • IV corticosteroids are administered every 4–6 hours, then are given orally. Continuous monitoring of client is critical Supplemental O2 is given by mask or nasal cannula for 90% O2 saturation. • Arterial catheter may be used to facilitate frequent ABG monitoring IV fluids are given because of insensible loss of fluids. • • • • CO2 narcosis • O2 toxicity • Absorption atelectasis • Infection Chronic O2 therapy at home improves • Prognosis. • Mental acuity. • Exercise tolerance • Hematocrit. • Pulmonary hypertension. Surgical therapy • Lung volume reduction surgery • Remove diseased lung to enhance performance of remaining tissue • Lung transplantation • Single lung—most common because of donor shortages • Prolongs life • Improves functional capacity • Enhances quality of life Breathing exercises • Decreases dyspnea, improves oxygenation, and slows respiratory rate • Pursed-lip breathing • Prolongs exhalation and prevents bronchiolar collapse and air trapping • Diaphragmatic breathing • Focuses on using the diaphragm instead of accessory muscles to achieve maximum inhalation and to slow the respiratory rate • • • Lack of evidence to support or refute this practice Effective coughing • Main goals • Conserve energy • Reduce fatigue • Facilitate removal of secretions Nutritional therapy • Weight loss and malnutrition are common among those with COPD. • Body mass index (BMI) between 21 and 25 kg/m2 • Rest (30 min.) before eating • Use a bronchodilator before meals. • 5–6 small meals a day • 1.2–1.3 times normal kilocalorie requirements to maintain weight • High-calorie, high-protein diet • 2–3 L fluid intake per day, taken between meals • Cold foods may cause less fullness than hot foods. • Avoid • Foods that require a great deal of chewing • Exercises and treatments 1 hour before and after eating • Gas-forming foods Hypertension Hypertension • description Persistent elevation of • Syst olic blo od pres sure Etiology and pathophysiology Clinical manifestations complications Diagnostic studies Nursing management • • Isolated systolic hypertension ≥14 0 mm Hg or Dia stoli c blo od pres sure ≥90 mm Hg or Cur rent use of anti hyp erte nsiv e med icati on(s ) • • Primary hypertension • • Sustained elevation of SBP ≥ 140 mm Hg and a DBP < 90 mm Hg Common in older adults related to loss of elasticity in large arteries 90% to 95% of clients Elevated BP without an identified cause • • T h e r e c o u l d b e s e v e r a l c o n t r Contributing factors • • • ↑ S N S a c t i v i t y ↑ S o d i u m r e t a i n i n • • Hypertension is a silent disease. Frequently it is asymptomatic until it becomes severe and target-organ disease has occurred. Secondary symptoms with severe hypertension include fatigue, reduced activity tolerance, dizziness, palpitations, angina, and dyspnea. • Hypertensive heart disease • • • • C o r o n a r y a r t e r y d i s e a s e L e f t v e n • • • Urinalysis Blood chemistry (potassium , sodium, blood urea, and creatinine) Fasting blood glucose Fasting total cholesterol and highdensity lipoprotein cholesterol , lowdensity lipoprotein cholesterol , and triglycerid es Standard 12-lead electrocar diography • • • Risk stratification Lifestyle modifications • Nutritional therapy • Weight reduction • Modification in alcohol consumption • Physical activity • Avoidance of tobacco products • Stress management Nursing assessment • Subjective data • I m p o r t a n t h e a i b u t i n g f a c t o r s • Focus on primary related to prevalence in clinical practice • • • g h o r m o n e s a n d v a s o c o n s t r i c t o r s ↑ S o d i u m i n t a k e D i a b e t e s m e l l i t u s > I d e a l b o d y w e i g h t • • • • t r i c u l a r h y p e r t r o p h y • H e a r t f a i l u r e Cerebro-vascular disease Peripheral vascular disease Nephrosclerosis Retinal damage • • • • • Assess urinary albumin excretion in clients with diabetes All clients with treated hypertensi on need to be monitored for the appearanc e of diabetes Ambulator y blood pressure monitorin g Useful in the diagnosis of uncomplic ated mildtomoderate hypertensi on Helpful in clients with suspected white coat hypertensi on, masked hypertensi on, apparent drug resistance, hypotensiv e symptoms with hypertensi ve medicatio ns, episodic hypertensi on, or autonomic nervous system dysfunctio n . • l t h i n f o r m a t i o n S y m p t o m s : D y s p n e a , f a t i g u e , i n t e r m i t t e n t c l a u d i c a t i o n , n o c t u r i a , • Risk factors • • • • • • • • • • • • • Patho • • • • • • • Advancing age Family history Heavy alcohol consumption Obesity Cigarette smoking Ethnicity Diabetes mellitus Sedentary lifestyle Elevated serum lipids Socioeconomic status High dietary sodium Psychosocial stress Gender Genes Sodium and water retention Altered renin–angiotensinaldosterone mechanism Stress and increased sympathetic nervous system activity Insulin resistance and hyperinsulinemia Endothelial cell dysfunction Obesity E x c e s s i v e a l c o h o l i n t a k e d i z z i n e s s , e r e c t i l e d y s f u n c t i o n • Objective data • C a r d i o v a s c u l a r : B P , p u l s e • M u s c u l o s k e l e t a l : T r u • • • • • Nursing diagnoses Planning Overall goals • Achieve and maintain target BP. • Understand and implement therapeutic plan. • Minimal or no unpleasant adverse effects n c a l o b e s i t y N e u r o l o g i c a l : M e n t a l s t a t u s c h a n g e s P o s s i b l e f i n d i n g s • • Confident of ability to manage and cope with condition Nursing implementation • Health promotion • • • I n d i v i d u a l c l i e n t e v a l u a t i o n S c r e e n i n g p r o g r a m s C a r d i o v a s c u l a r r i s k f a c t o r m o d i f i c a t i o n • Ambulatory and home care • P h y s i c a l a c t i v i t y • H o m e b l o o d p r e s s u r e m o n i t o r i n g • C l i e n t a d h e r e n c e • Secondary hypertension • 5% to 10% in adults; >80% in children Many causes; treatment aimed at eliminating the underlying cause • Causes • • • • • • • Hypertensive crisis • • • Severe, abrupt increase in DBP (defined as >120–130 mm Hg) Rate of increase in BP is more important than the absolute value. Often occurs in clients with a history of hypertension who have failed to comply with medications or who have been undermedicated Evaluation Coarctation or congenital narrowing of the aorta Renal disease such as renal artery stenosis and parenchymal disease Endocrine disorders such as pheochromocyt oma, Cushing’s syndrome, and hyperaldosteron ism Neurological disorders such as brain tumours, quadriplegia, and head injury Sleep apnea Medications Pregnancyinduced hypertension • Hypertensive emergency = evidence of acute target organ damage • Hy pert ensi ve enc eph alo pat hy, cere bral he mor rha ge • Acu te ren al fail ure • My oca rdia l infa rcti on • Acu te left ven tric ular • • • • • IV drug therapy: titrated to mean arterial pressure Monitor cardiac and renal function Neurological checks Determine cause Education to avoid future crises • fail ure wit h pul mo nar y ede ma Dis sect ing aort ic ane ury sm Coronary artery disease Coronary Artery Disease Description Atherosclerosis: type of blood vessel disorder Begins as soft deposits of fat that harden with age Referred to as “hardening of arteries” Can occur in any artery in the body Atheromas (fatty deposits) Preference for the coronary arteries Etiology & Pathophysiology Atherosclerosis is the major cause of CAD. Characterized by a focal deposit of lipids, primarily within the intimal wall of the artery Endothelial lining altered as a result of inflammation and injury. C-reactive protein (CRP) Nonspecific marker of inflammation Increased in many clients with CAD Chronic elevation of CRP is associated with unstable plaques and oxidation of LDL cholesterol Developmental stages: fatty streaks Earliest lesions Characterized by lipid-filled smooth muscle cells Potentially reversible Developmental stages: fibrous plaque Beginning of progressive changes in the arterial wall Lipoproteins transport cholesterol and other lipids into the arterial intima. Fatty streak is covered by collagen, forming a fibrous plaque that appears grayish or whitish. Result = narrowing of vessel lumen Developmental stages: complicated lesion Continued inflammation can result in plaque instability, ulceration, and rupture. Risk Factors Nonmodifiable Risk Factors • Age • Gender • Ethnicity • Family history • Genetic predisposition Modifiable risk factors • Elevated serum lipids • Hypertension • Tobacco use • Physical inactivity • Obesity Modifiable Contributing Risk Factors • Diabetes • Metabolic syndrome • Psychological states • Homocysteine level • Substance use Health Promotion / Healing process • Identification of people at high risk • Health history, including use of prescription/nonprescription medications • Presence of cardiovascular symptoms • Environmental patterns: diet, activity • Psychosocial history • Values and beliefs about health and illness • Health-promoting behaviours • Physical fitness • FITT formula: 30 minutes on most days of the week • Regular physical activity contributes to • weight reduction. • reduction of systolic BP. • in some men more than women, increase in HDL cholesterol. • • Platelets accumulate and thrombus forms. Increased narrowing or total occlusion of lumen Collateral circulation Normally, some arterial anastomoses (or connections) exist within the coronary circulation. Growth and extent of collateral circulation are attributed to two factors. • Inherited predisposition to develop new vessels (angiogenesis) • Presence of chronic ischemia When occlusion of the coronary arteries occurs slowly over a long period, the chance that adequate collateral circulation will develop is greater. Acute Coronary Syndrome • • When ischemia is prolonged and is not immediately reversible, acute coronary syndrome (ACS) develops. ACS encompasses • Unstable angina (UA) • Non–ST-segmentelevation myocardial infarction (NSTEMI) • ST-segmentelevation MI (STEMI) Deterioration of once-stable plaque -> Rupture -> Platelet aggregation -> Thrombus • • • Result Partial occlusion of coronary artery: UA or NSTEMI Total occlusion of coronary artery: STEMI • • • • • • Health education in schools Nutritional therapy • Therapeutic lifestyle changes • Omega-3 fatty acids Health-promoting behaviours • Cholesterol-lowering drug therapy • Drugs that restrict lipoprotein production: statins • Drugs that increase lipoprotein removal: bile acid sequestrants • Drugs that decrease cholesterol absorption: Ezetimibe (Ezetrol) • Antiplatelet therapy • ASA • Clopidogrel (Plavix) Within 24 hours, leukocytes infiltrate the area of cell death. Enzymes are released from the dead cardiac cells (important indicators of MI). Proteolytic enzymes of neutrophils and macrophages remove all necrotic tissue by second or third day. Development of collateral circulation improves areas of poor perfusion. Necrotic zone is identifiable by ECG changes and nuclear scanning. 10–14 days after MI, scar tissue is still weak and vulnerable to stress. By 6 weeks after MI, scar tissue has replaced necrotic tissue. • Area is said to be healed, but less compliant. Ventricular remodelling • Normal myocardium will hypertrophy and dilate in an attempt to compensate for the infarcted muscle. Sudden Cardiac Death • • Unexpected death from cardiac causes Rapid CPR, defibrillation with AED, and early advanced cardiac life support increase survival rates. • • • • Syncope • Brief lapse in consciousness accompanied by a loss in postural tone (fainting) • • Abrupt disruption in cardiac function, resulting in loss of CO and cerebral blood flow Death usually within 1 hour of onset of acute symptoms (e.g., angina, palpitations) Most SCDs are caused by ventricular dysrhythmias (e.g., ventricular tachycardia). SCD occurs less commonly as a result of LV outflow obstruction (e.g., aortic stenosis). Primary risk factors • Left ventricular dysfunction • Ventricular dysrhythmias following MI • • • • • • • • • Diagnostic workup to rule out or confirm MI • Cardiac markers • ECG Cardiac catheterization PCI or CABG 24-hour Holter monitoring Exercise stress testing Signal-averaged ECG Electrophysiologic study (EPS) Implantable cardioverter-defibrillator (ICD) Psychosocial adaptation • “Brush with death” • “Time bomb” mentality • Possible role changes • Driving restrictions • Change in occupation Cardiovascular causes • Neurocardiogenic syncope or “vasovagal” syncope (e.g., carotid sinus sensitivity) • Primary cardiac dysrhythmias (e.g., tachycardias, bradycardias) Noncardiovascular causes • Hypoglycemia • Hysteria • Unwitnessed seizure • Vertebrobasilar transient ischemic attack Anginas Chronic Stable Angina Etiology & Pathophysiology Reversible (temporary) myocardial ischemia = angina (chest pain) • O2 demand > O2 supply • Primary reason for insufficient blood flow is narrowing of coronary arteries by atherosclero sis. • Referred pain in left Clinical Manifestations • Pain usually lasts 3–5 minutes. • Subsides when the precipitating factor is relieved. • Pain at rest is unusual. • ECG reveals ST-segment depression and/or T- Types of Anginas / Complications Silent ischemia • Ischemia that occurs in the absence of any subjective symptoms • Associated with diabetic neuropathy • Confirmed by ECG changes Nocturnal angina • Occurs only at night but not necessarily during sleep Prinzmetal’s (variant) angina Occurs at rest usually in response to spasm of major coronary artery Diagnostic Studies Health history/physical examination Laboratory studies 12-lead ECG Chest x-ray Echocardiogram Exercise stress test Cardiac catheterization/coronary angiography Diagnostic Coronary revascularization: percutaneous coronary intervention (PCI) Balloon angioplasty Stent Nursing and Collaborative Management • Drug therapy: goal: ↓ O2 demand and/or ↑ O2 supply • Short-acting nitrates: sublingual • Long-acting nitrates • Nitroglycerin (NTG) ointment • Transdermal controlledrelease NTG • β-Adrenergic blockers • Calcium channel blockers • If β-adrenergic blockers are poorly tolerated, contraindicated, or do not control angina Unstable Angina (Myocardial Infarction) • • • • • shoulder and arm is from transmission of the pain message to the cardiac nerve roots. • Intermittent chest pain that occurs over a long period with the same pattern of onset, duration, and intensity of symptoms Result of sustained ischemia (>20 minutes), causing irreversible myocardial cell death (necrosis) Necrosis of entire thickness of myocardium takes 5–6 hours The degree of altered function depends on the area of the heart involved and the size of the infarct. Contractile function of the heart is disrupted in areas of myocardial necrosis. Most MIs involve the left ventricle (LV). • wave inversion. Pain described as • Constrictive • Squeezing • Heavy • Choking • Suffocating • Seen in clients with a history of migraine headaches and Raynaud’s phenomenon Spasm may occur in the absence of CAD. When spasm occurs • Used to manage Prinzmetal’s angina Angiotensin-converting enzyme inhibitors Chest pain Marked, transient ST-segment elevation May occur during REM sleep May be relieved by moderate exercise or may disappear spontaneously Microvascular angina Myocardial ischemia secondary to microvascular disease affecting the small, distal branches of the coronary arteries More common in women Triggered by ADLs • • Pain • • Total occlusion → anaerobic metabolism and lactic acid accumulation → severe, immobilizing chest pain not relieved by rest, position change, or nitrate administratio n • Described as heaviness, constriction, tightness, burning, pressure, or crushing • Common locations: substernal, retrosternal, or epigastric areas; pain may radiate to neck, jaw, arms, back Stimulation of sympathetic nervous system results in • Release of glycogen • Diaphoresis • Vasoconstrict ion of • • Dysrhythmias • Most common complication • Present in 80% of MI clients • Most common cause of death in the pre-hospital period • Lifethreatening dysrhythmias seen most often with anterior MI, heart failure, or shock Heart failure • A complication that occurs when the pumping power of the heart has diminished Cardiogenic shock • Occurs when inadequate oxygen and nutrients are supplied to the tissues because of severe LV failure • • • • • • • • Detailed health history and physical 12-lead ECG: changes in QRS complex, ST segment, and T wave can rule out or confirm UA or MI. Serum cardiac markers Coronary angiography Others: exercise stress testing, echocardiogram Definitive ECG changes occur in response to ischemia, injury, or infarction of myocardial cells. • Changes seen in the leads that face the area of involvement Ischemia • ST segment depression and/or T wave inversion • ST segment depression is significant if it is at least 1 mm (one small box) below the isoelectric line. • Changes occur in response to the electrical disturbance in myocardial cells due to inadequate supply of oxygen. • Once treated (adequate blood flow is restored), ECG changes resolve and ECG returns to baseline. Injury • ST-segment elevation is significant if >1 mm above the isoelectric line. • If treatment is prompt and effective, may avoid infarction • I f • • • • • Initial Management • Ensure patent airway • Administer O2 by nasal cannula or NRB • Obtain 12-lead ECG • Insert two IV-catheters • Assess pain using PQRST • Medicate for pain as ordered • Initiate continuous ECG monitoring and determine underlying rhythm • Obtain blood test results • Obtain CXR • Assess for antiplatelet, anticoagulation, or fibrinolytic therapy or PCI as appropriate • Administer ASA and beta-adrenergic blockers for cardiac-related CP • Administer antidysrhythmic medications as ordered Ongoing Management • Monitor vital signs, LOC, cardiac rhythm and O2 saturation • Monitor response to medications • Provide reassurance and emotional support to patient/family • Explain all interventions and procedures • Anticipate need for intubation if respiratory distress is evident • Prepare for CPR, defibrillation, TCP or cardioversion Emergent PCI • Treatment of choice for confirmed MI • Balloon angioplasty + drug-eluting stent(s) Fibrinolytic therapy • Indications and contraindications • Best marker of reperfusion: return of ST segment to baseline • Rescue PCI if thrombolysis fails. • Major complication: bleeding Drug therapy • • • peripheral blood vessels • Skin: ashen, clammy, and/or cool to touch Cardiovascular • Initially, ↑ HR and BP, then ↓ BP (secondary to ↓ in CO) • Crackles • Jugular venous distension • Abnormal heart sounds • S 3 o r S 4 • N e w m u r m u r Nausea and vomiting • Can result from reflex stimulation of the vomiting centre by severe pain Fever • Systemic manifestation of the inflammatory process caused by cell death • • • • • Requires aggressive management Papillary muscle dysfunction • Causes mitral valve regurgitation • Condition aggravates an already compromised LV Ventricular aneurysm • Results when the infarcted myocardial wall becomes thinned and bulges out during contraction Acute pericarditis • An inflammation of visceral and/or parietal pericardium • May result in cardiac compression, ↓ LV filling and emptying, heart failure • Pericardial friction rub may be heard on auscultation. • Chest pain different from MI pain Dressler syndrome • Characterized by pericarditis with effusion and fever that develop 4–6 weeks after MI • Pericardial (chest) pain • Pericardial friction rub may be heard on auscultation. • Arthralgia s e r u m c a r d i a c m a r k e r s a r e p r e s e n t , a n S T s e g m e n t – e l e v a t i o n m y o c a r d i a l • • • • • • • • Intravenous nitroglycerin Morphine sulphate Beta-adrenergic blockers Angiotensin-converting enzyme inhibitors • Antidysrhythmic medications • Cholesterol-lowering drugs • Stool softeners • Anticoagulation Nutritional therapy • Initially NPO • Progress to • Low salt • Low saturated fat • Low cholesterol Coronary surgical revascularization • Failed medical management • Presence of left main coronary artery or three-vessel disease • Not a candidate for PCI (e.g., lesions are long or difficult to access) • Failed PCI with ongoing chest pain • History of diabetes mellitus Coronary surgical revascularization • Coronary artery bypass graft (CABG) surgery • Requires sternotomy and cardiopulmonary bypass (CPB) • Uses arteries and veins for grafts • Minimally invasive direct coronary artery bypass (MIDCAB) • Alternative to traditional CABG Coronary surgical revascularization • Off-pump coronary artery bypass • Does not require CPB • Transmyocardial laser revascularization • For clients with advanced CAD who are not surgical candidates or who have failed maximum medical therapy i n f a r c t i o n ( S T E M I ) h a s o c c u r r e d . • • Infarction • Physiological Q wave is the first negative deflection following the P wave. • Small and narrow (<0.04 second in duration) • Pathological Q wave is deep and >0.03 second in duration. • Pathological Q wave indicates that at least half the thickness of the heart wall is involved. • Referred to as a Q-wave MI • Pathological Q wave may be present indefinitely • T-wave inversion related to infarction occurs within hours and may persist for months. After an MI, certain proteins called serum cardiac markers are released into the blood in large quantities from necrotic heart muscle • Troponin • • CK-MB Myoglobin Vascular disorders Arterial Disorders Peripheral Artery Disease Description • • • Acute arterial ischemic Aortic Anyerism Aortic Disection disorders Peripheral arterial • Acute arterial • One of the most Aortic disease (PAD) is a ischemia is a common problems dissection progressive narrowing sudden affecting the aorta is occurs most and degeneration of the interruption in an aneurysm, a commonly in arteries of the upper and the arterial permanent, localized the thoracic lower extremities. blood supply to outpouching or aorta and is • In most cases, it is tissue, an dilation of the vessel the result of a a result of organ, or an wall (either congenital tear in the atherosclerosis. extremity that, or acquired). intimal Although the exact if left • Aortic aneurysms may (innermost) cause(s) of untreated, can involve the aortic arch, lining of the atherosclerosis are result in tissue thoracic aorta, and/or arterial wall unknown, inflammation death. abdominal aorta, but allowing blood and endothelial injury • Common most are found in the to “track” play a major role. causes include: abdominal aorta between the The most significant risk • Embolis below the level of the intima and factors for PAD are m renal arteries. media and tobacco use, diabetes, • Thromb • The primary causes of creates a false hyperlipidemia, elevated osis aortic aneurysms can lumen of high-sensitivity C• Trauma. be classified as blood flow. reactive protein, and degenerative, • Most experts uncontrolled congenital, attribute hypertension. mechanical, nontraumatic aortic • • The most common locations for PAD are the carotid, common iliac, superficial femoral, popliteal, and tibial arteries. PAD of the lower extremities affects the aortoiliac, femoral, popliteal, tibial, or peroneal arteries. • • • Clinical Manifestati ons • The classic symptom of PAD of the lower extremities is intermittent claudication, which is defined as ischemic muscle ache or pain that • • Specific manifestations depend on the affected area of the body. Signs and symptoms of an • inflammatory, or infectious Classified as true or false A true aneurysm is one in which the wall of the artery forms the aneurysm, with at least one vessel layer still intact • Further subdivided into fusiform and saccular dilations A false aneurysm (or pseudoaneurysm) is not an aneurysm but a disruption of all layers of the arterial wall, resulting in bleeding that is contained by surrounding structures Thoracic aortic aneurysms: • Often asymptomatic • Deep, diffuse chest pain that may extend to • dissection to the degeneration of the elastic fibres in the medial layer. Chronic hypertension accelerates the degradation process. A sudden, severe pain in the anterior part of the chest Intrascapular pain radiating • • is precipitated by a consistent level of exercise, resolves within 10 minutes or less with rest, and is reproducible. Paresthesia, manifested as numbness or tingling in the toes or feet, may result from nerve tissue ischemia. Gradually diminishing perfusion to neurons obviates sensations of both pressure and deep pain. Thus, affected patients may not notice lower extremity injuries. Physical findings include thin, shiny, and taut skin; loss of hair on the lower legs; diminished or absent pedal, popliteal, or femoral pulses; pallor or blanching of the foot in response to leg elevation (elevation pallor); and reactive hyperemia (redness of the foot) when the limb is in a dependent acute arterial ischemia usually have an abrupt onset and include the “six Ps:” • Pain • Pallor • Pulseles sness • Paresth esia • Paralysis • Poikilot hermia (adaptat ion of the ischemic limb to its environ mental tempera ture, most often cool). • the intrascapular area • Hoarseness as a result of pressure on the recurrent laryngeal nerve • Dysphagia from pressure on the esophagus. Abdominal aortic aneurysms (AAAs): • Often asymptomatic • Symptoms may mimic pain associated with abdominal or back disorders. down the spine into the abdomen or legs. The pain is most frequently described as “sharp” and “worst ever” followed by “tearing” or “ripping.” • Complicatio ns • • • Diagnostic studies • position (dependent rubor). Rest pain most often occurs in the forefoot or toes, is aggravated by limb elevation, and occurs when there is insufficient blood flow to maintain basic metabolic requirements of the tissues and nerves of the distal extremity. Complications of PAD include nonhealing ulcers over bony prominences on the toes, feet, and lower leg, and gangrene. Amputation may be required if blood flow is not restored. Critical limb ischemia is a chronic condition characterized by ischemic rest pain, arterial leg ulcers, and/or gangrene of the leg due to advanced PAD. • The most serious complication related to an untreated aneurysm is rupture and bleeding. Tests used to diagnose PAD include Doppler • Chest radiograph • Diagno stic • • • ultrasound with segmental blood pressures at the thigh, below the knee, and at ankle level. A drop in segmental BP of more than 30 mm Hg indicates PAD. Ankle-brachial index Angiography is used to delineate the location and extent of the disease process. • • • Electrocardiogram (to rule out myocardial infarction) Echocardiography CT scan Magnetic resonance imaging (MRI) scan • • • • • • Collaborati ve Care • Collaborative care • Risk factor modification • Drug therapy • Exercise therapy • Nutritional therapy • Complementary and alternative therapies • • • • • Anticoagulation Thrombolysis Embolectomy Surgical revascularizatio n Amputation • • The goal of management is to prevent the aneurysm from rupturing and extension of dissection. Surgical repair of AAA involves 1. Incising the diseased • • • studies used to assess aortic dissecti on are similar to those perfor med for AAA. Health history CXR CT scan ECG MRI TEE Conser vative therapy Endova scular dissecti on repair Surgical therapy • • Care of the leg with critical limb ischemia • Interventional radiology catheter-based procedures • Surgical therapy Nursing Implementation, Acute Intervention • Post operatively: • Check the extremity q15 minutes for colour, temperatu re, capillary refill, presence of peripheral pulses, and sensation and movemen t (CSM) • Notify physician • • segment of the aorta 2. Removing thrombus or plaque 3. Inserting a synthetic graft 4. Suturing the native aortic wall around the graft. Minimally invasive endovascular aneurysm repair (EVAR) is an alternative to conventional surgical repair of AAA and involves the placement of a sutureless aortic graft into the abdominal aorta inside the aneurysm via a femoral artery cutdown. Nursing implementation 1. Health promotion 2. Acute intervention • Preoperatively • Keep the client in bed in a semiFowler’ s positio n and maintai na quiet environ ment, to help keep the systolic BP at the lowest possibl e level that maintai ns vital organ perfusi on • • if no pulses present Monitor for bleeding, hematom a, thrombosi s, embolizati on, and compartm ent syndrome Dramatic increase in pain, loss of palpable pulses, extremity pallor or cyanosis, numbness /tingling, cold extremity suggests occlusion • • • • • • • Graft patency Cardiov ascular status Infectio n Gastroi ntestina l status Neurolo gical status Periphe ral perfusi on status Renal perfusi on status • (typical ly betwee n 110 and 120 mm Hg) • Opioids and sedativ es are admini stered as ordere d. Postoperativel y • Client and caregiv er teachin g • Followup with regularl y schedul ed • • • of graft or stent Do not flex knee POD #1 – out of bed and in chair Monitor for infection • magnet ic resona nce imagin g (MRI) or comput ed tomogr aphy (CT) All clients with a history of aortic dissecti on, regardl ess of anatom ical locatio n or treatm ent modalit y, require longterm medica l therapy to control BP. Venous Disorders Venous thrombosis Description • • • • Venous thrombo-embolism (VTE), also known as venous thrombosis, is the most common disorder of the veins and involves the formation of a thrombus (clot) in association with inflammation of the vein. Superficial vein thrombosis (SVT) is the formation of a thrombus in a superficial vein Deep vein thrombosis (DVT) involves a thrombus in a deep vein, most commonly the iliac and femoral veins, and can result in embolization of thrombi to the lungs. VTE represents the spectrum of pathology from DVT to pulmonary embolism. Varicose Veins • • • • Varicose veins, or varicosities, are dilated, tortuous subcutaneous veins most frequently found in the saphenous vein system. Primary varicose veins are more common in women and patients with a strong family history and are probably caused by congenital weakness of the veins. Secondary varicose veins typically result from a previous VTE. Secondary varicose veins also may occur in the Chronic venous insufficiency and venous leg ulcers • Chronic venous insufficiency (CVI) is a condition in which leg veins and valves fail to keep blood moving forward. • This results in ambulatory venous hypertension. • CVI often occurs as a result of previous episodes of VTE and can lead to venous leg ulcers. • It is a common medical problem in older adults. • • • • Three important factors (called Virchow’s triad) in the etiology of venous thrombosis are • Venous stasis • Damage of the endothelium (inner lining of the vein) • Hypercoagulability of the blood. Localized platelet aggregation and fibrin entrap RBCs, WBCs, and more platelets to form a thrombus A frequent site of thrombus formation is the valve cusps of veins, where venous stasis occurs As the thrombus enlarges, increased numbers of blood cells and fibrin collect behind it, producing a larger clot with a “tail” that eventually occludes the lumen of the vein • esophagus, vulva, spermatic cords, and anorectal area, and as abnormal arteriovenous connections. Reticular veins are smaller varicose veins that appear flat, less tortuous, and blue-green in colour. Risk factors include • Family history of chronic venous disease • Congenital weakness of the vein structure • Female sex • Use of oral contraceptives or hormone therapy • Latin American ethnicity • Increasing age • Obesity • Multiparity • Venous obstruction resulting from thrombosis or extrinsic pressure by tumours • Occupations that require prolonged standing or sitting. • • • Causes of CVI include: • Vein valve incompetence • Deep vein obstruction • Congenital venous malformation • Arteriovenous fistula The basic problem is incompetent valves of the deep veins. Hydrostatic pressure in veins increases and serous fluid + RBCs leak from capillaries and venules into the tissue edema Superficial vein thrombosis Clinical • May have a palpable, firm, Manifestations subcutaneous cordlike vein • Area surrounding the vein may be tender to the touch, reddened, and warm • A mild systemic temperature elevation and leukocytosis may be present Venous thrombo-embolism • The patient with VTE may or may not have: • Unilateral leg edema • Extremity pain • A sense of fullness in the thigh or calf • Paresthesias • Warm skin • Erythema • A systemic temperature greater than 38oC. • Positive Homan’s sign (pain on forced dorsiflexion of the foot when the leg is raised) Complications VTE • • Pulmonary embolism (PE) Chronic venous insufficiency Chronic venous insufficiency (CVI) results from valvular • • • Ache or pain after prolonged standing • Relieved by walking or by elevating the limb. Pressure, itchy, burning or cramp-like sensation Swelling and/or nocturnal leg cramps in the calf may occur. • • • • Over time, the skin and subcutaneous tissue around the ankle are replaced by fibrous tissue, resulting in thick, hardened, contracted skin. The skin of the lower leg is leathery, with a characteristic brownish or “brawny” appearance from the hemosiderin deposition (break down of RBCs). Edema and eczema, or “stasis dermatitis,” are often present, and pruritus (itching) is a common complaint. The wound margins are irregularly shaped, and the tissue is typically a ruddy colour. • • Diagnostic studies destruction, allowing retrograde flow of venous blood. Phlegmasia cerulea dolens (rare complication) Post-thrombotic syndrome results from chronic venous hypertension caused by valvular destruction, stiffness and noncompliance • VTE • • • • • • • Clinical assessment Blood work D-dimer testing Ultrasonography Computed tomography venography Magnetic resonance venography Contrast venography • • • • • • • Treatment usually is not indicated if varicose veins are only a cosmetic problem Rest with the affected limb elevated Compression stockings Exercise, such as walking Sclerotherapy Laser therapy/highintensity pulsed-light therapy Surgical intervention Prevention is key. • Client should be instructed to avoid sitting or standing for long periods, maintain ideal body weight, take precautions against injury to the • • Collaborative Care Superficial vein thrombosis • Immediate removal of the IV catheter • If edema is present, the extremity should be elevated to promote reabsorption of fluid from the interstitial space into the vasculature • Application of warm, moist heat may hep relieve pain and inflammation • Oral NSAIDs • Compression Venous thrombo-embolism • Prevention and prophylaxis • Drug therapy extremities, avoid wearing constrictive clothing, and walk daily. After vein ligation surgery • Client should be encouraged to deep breathe, to promote venous return. Long-term management • Improving circulation, relieving comfort, avoiding complications • Compression • Elastic wraps, custom-fitted compression stockings, elastic tubular support bandages, Velcro wrap, SCDs, paste bandage with an elastic wrap and multilayer (three or four) • • • Vitamin K antagonsists (ie. warfarin) • Thrombin inhibitors: indirect • Thrombin inhibitors: direct • Factor Xa inhibitors • Anticoagulation therapy for VTE prophylaxis • Anticoagulation therapy for venous thromboembolism treatment Thrombolytic therapy for venous thromboembolism Surgical therapy • • • • • • • • bandage system Moist environment dressings Routine antibiotic therapy is not indicated Monitor for signs of infection Nutritional support Maintaining normal blood glucose levels Long-term management of venous leg ulcers should focus on teaching the client about self-care measures because the ulcers often recur. Instruct the client and caregiver to avoid trauma to the limbs and teach them proper skin care. Demonstrate the correct application of graduated compression stockings and stress the importance of regular replacement (every 4–6 months). Condition Dysrhythmias All rhythms Coronary Artery Disease Chronic Stable Angina Unstable Angina NSTEMI STEMI Associated Lab Values to Consider Rationale for Considering Potassium Potassium impairs repolarization process High potassium = peaked T waves, widened QRS when severe Low potassium = flattened T wave, U wave Troponin CK-MB Myoglobin Heart Failure BNP Hypertension Inflammatory Conditions Infective Endocarditis Acute Pericarditis Rheumatic Fever Valvular Heart Disease Cardiomyopathy Peripheral Arterial Disease PAD Blood cultures Acute Arterial Ischemia Raynaud’s Aortic Aneurysm Venous Disorders Venous thromboembolism Varicose Veins Venous Insufficiency General Diagnostics Diagnostic/Lab Test Central venous pressure (CVP) D-Dimer Normal 2-9 mm Hg High = right ventricular failure, volume overload Low = hypovolemia Hematocrit Men 0.42-0.52 Women 0.37-0.47 High = dehydration Low = anemia Hemoglobin WBC count WBC differential Platelet count Activated partial thromboplastin time (aPTT) Purpose Measurement of preload and can be used to monitor the pressure in the right atrium and right ventricle. The CVP reading is influenced by the function of the left side of the heart, pressures in the pulmonary vessels, venous return to the heart, and the position of the patient when the reading is taken. Measurement of packed cell volume of RBCs. Hematocrit is increased in chronic hypoxemia. Value is expressed as a percentage of the total blood volume. d-Dimer International Normalized Ratio (INR) Erythrocyte sedimentation rate (ESR) Erythropoietin Chest XRAY CT Scan MRI Positron emission tomography (PET) Albumin Wound cultures Blood cultures Cardiac Diagnostic/Lab Test MB isoenzyme of creatine kinase (CK-MB) Troponin Myoglobin C-Reactive protein B-type natriuretic peptide (BNP) Cholesterol Triglycerides Lipoproteins (HDL, LDL) ECG Holter monitor Exercise stress test Echocardiography Normal Purpose Stress echocardiography Transesophageal echocardiography (TEE) Multigated acquisition (MUGA) Cardiac catheterization Coronary angiography Endocrine Diagnostic/Lab Test Thyroid stimulating hormone (TSH) Thyroxine (T4) total Triiodothyronine (T3) Free T4 Parathyroid hormone Total serum calcium Ionized calcium Serum phosphate Cortisol (blood + urine) Aldosterone (blood + urine) Adrenocorticotrophic hormone (ACTH) Normal Purpose Fasting blood glucose level (FBG) Oral glucose tolerance test (OGTT) Capillary glucose monitoring Glycosylated hemoglobin (A1C) Glucose (urine) Ketones (urine) Anion gap 8-16 mmol/L High anion gap = acid gain Difference between the measured serum cations and anions in ECF Na – (HCO3 + Cl) Microalbumin Respiratory Diagnostic/Lab Test Arterial blood gas Venous blood gas Oximetry Bronchoscopy Lung biopsy Thoracentesis Pulmonary function test Ventilation-perfusion (VQ) scan Sputum culture and sensitivity Sputum gram stain Acid-fast smear and culture Cytology study Normal Purpose