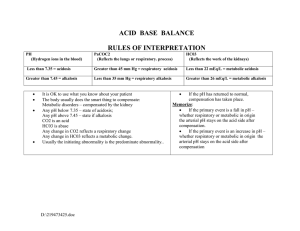
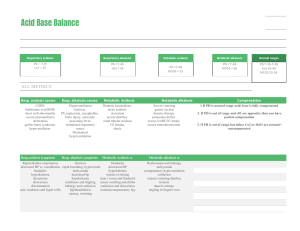
ACID-Base Balance 20206 8/2022 Purpose: Acid-Base Balance • Maintain a steady balance between acids and bases to achieve homeostasis • Symptom of underlying health problems • Health problems lead to imbalances • Diabetes • Vomiting/diarrhea • Respiratory conditions • Kidney disease • Acid excretion systems: Lungs & Kidneys Acid-Base Balance • Lungs excrete carbonic acid (CO2 & H2O) • Byproducts of cellular metabolism • Medulla controls excretion rate • Kidneys excrete metabolic acids (HCO3) • Excretes acidic urine • Compensates by reabsorbing HCO3 and excreting more H+ pH: Acid-Base Balance Measure of Hydrogen (H+) ion concentration pH neutral range 7.35 - 7.45 pH lower than 7.35 is acidic and hydrogen ions are increasing pH higher that 7.45 is alkaline and hydrogen ions are decreasing pH: Acid-Base Balance • https://www.youtube.com/watch?v=w3nsxx6AcdA Water CO2 + H2O Carbon dioxide Bicarbonate ion H2CO3 Carbonic acid HCO3 + H Hydrogen ion Acid Base Balance Types CO2 + H2O H2CO3 Respiratory Acidosis – pH decrease • Respiratory Acidosis - CO2 • Can’t get air out • COPD, asthma, pulmonary edema • Respiratory Alkalosis - CO2 • Breathing too fast • Anxiety, pain, hyperventilating, pregnancy HCO3 + H Metabolic Alkalosis – pH increase • Metabolic Acidosis - HCO3 or H+ • Too much acid • DKA, renal failure, diarrhea • Metabolic Alkalosis - HCO3 or H+ • Too little acid • Vomiting, NG suction, diuresis, ingestion of baking soda or antacids Respiratory Acidosis Alkalosis Alveolar hypoventilation Alveolar hyperventilation Excessive carbonic acid in blood Deficit of carbonic acid in blood stream stream Renal compensation begin to Treat reason for tachypnea work within 24 hours Renal compensation which chronic respiratory diseases • Pulmonary fibrosis Metabolic Acidosis Alkalosis Increase in metabolic acid and Increase in bicarbonate and decrease in bicarbonate decrease in metabolic acid Kidneys unable to excrete enough Respiratory rate decreases acid so it accumulates Decreased level of consciousness Kussmaul respirations (deep rapid) BUFFERS • https://www.youtube.com/watch?v=tC9EfkOe8IQ Water CO2 + H20 Carbon Dioxide Hydrogen ion H2CO3 H+ + HCO3 Carbonic Acid Bicarbonate ion (weak acid) (weak base) https://www.youtube.com/watch?v=5_S5wZks9v8 (different video but the same buffer system idea) Acidosis Signs & Symptoms • Lethargy, • Confusion • Dizziness • Headache • Low BP • Dysrhythmias • Respiration changes Alkalosis Signs & Symptoms • • • • • • • • • • • • Confusion Dizziness Lethargy Headache Light headedness Tachycardia Dysrhythmias Nausea/vomiting/diarrhea Anorexia/abd pain Tremors/tingling fingers Seizures Respiratory rate changes Acid-Base Imbalances Kidney & Lung compensate for each other Compensatory mechanisms do NOT correct the problem If underlying problem isn’t corrected, compensatory mechanisms will fail Nursing Process : Subjective Data • Past Medical History • Medications • Surgery or other treatments • Health perception • Nutritional-Metabolic pattern • Elimination pattern • Activity/Exercise regimen • Cognitive-Perceptual changes Nursing Process : Objective Data • Physical Exam • Daily weight • Accurate I&O • Laboratory Values What would be concerning? Nursing Diagnosis • Acute Confusion • Impaired gas exchange • Ineffective breathing pattern • Decreased cardiac output • Deficient fluid volume • Excessive fluid volume • Ineffective tissue perfusion • Impaired oral mucous membrane • Risk for injury • Deficient knowledge regarding disease management Etiology of Imbalances Recap • Respiratory Acidosis – hypoventilation, COPD, retaining CO2 • Respiratory Alkalosis – hyperventilation, blowing off too much CO2 • Metabolic Acidosis – kidney failure, DKA, shock • Metabolic Alkalosis – vomiting, NG suctioning ABG Arterial Blood Gas Provides information about: • Acid-base status • Underlying cause of imbalance • Compensation? • Overall oxygen status Interpreting ABG results Diagnosis in 5 steps 1. Evaluate pH (7.35-7.45) 2. Analyze PaCO2 (35-45) 3. Analyze HCO3 (22-26) 4. Determine if CO2 or HCO3 matches the alternation 5. Decide if the body is attempting to compensate https://www.youtube.com/watch?v=URCS4t9aM 5o Jeri is a 22-year-old female who has been on a 3-day party binge. Her friends bring her to ED because she will not wake up. Case Study 1 Assessment reveals shallow respirations with a rate of 8/min, diminished breath sounds, and decreased level of consciousness. 1. What type of acid-base imbalance would you expect? 2. What is causing it? 3. What type of compensation would you expect or not expect? • Maya is an 18-year-old female who presents to the ED after a sexual assault. She is hysterical and in severe emotional distress. • Her BP is 140/96, heart rate 104, respiratory rate 38, and oxygen saturation 96%. Lung sounds are clear. Case Study 2 1. What type of acid-base imbalance would you expect? 2. What is causing it? 3. What interventions can help her compensate? Case Study 3 Alan is a17 year old male who comes to the clinic complaining of fatigue, constant thirst, and frequent urination. Focused assessment reveals: rapid deep respirations (rate 28) with a fruity odor. Blood glucose is 484. 1. What type of acid-base imbalance would you expect Alan to have? 2. What is causing it? 3. What interventions would be started? Case Study 4 Anthony is a 54-year-old male with a history of nausea and vomiting for the past week. He has been self medicating himself with antacids to control his abdominal discomfort. What type of acid-base imbalance would you expect? What is causing it? What is the body’s compensatory response? What is the treatment? & Oral Fluid and Electrolyte Replacement Used to correct mild fluid and electrolyte deficits • • • • • Water Glucose Potassium Magnesium Sodium IV Fluid Replacement • Purpose: • Maintenance • When oral intake is not adequate • Replacement • When losses have occurred • Types of fluids categorized by tonicity Hypotonic – 0.45% Normal Saline • Lower osmolality compared to plasma • Dilutes ECF – moves fluid into cells • Can lower BP and can cause swelling Isotonic – D5, 0.9% NS, D5 0.25%NS, LR IV Fluids • Osmolality similar to plasma • Expands ECF and does NOT move into cells • May cause hypernatremia Hypertonic – D10, D5 0.45%NS, D5NS, • Higher osmolality compared to plasma • Draws fluids out of ECF • May cause intravascular fluid volume excess • Treats hyponatremia & head trauma injuries D5W • Dextrose quickly metabolizes • Provides 50g of Dextrose/Liter or 170 calories/Liter • Used to replace water loss, helps prevent ketosis associated with starvation Normal Saline (NS) • Sodium concentration slightly higher than plasma • Chloride concentration significantly higher than plasma • Many cause hypernatremia & hyperchloremia • Good for patient with vomiting & diarrhea • Only solution used with blood transfusions Lactated Ringer’s Solution (LR) • Contains sodium, potassium, chloride, and lactate • Same concentration as ECF • Fluid for surgery, burns, GI fluid loss, • Do NOT give to: liver dysfunction, hyperkalemia, severe hypovolemia because they have a decreased ability to convert the lactate to bicarb Dextrose 10% • Provides 340 calories/Liter • Provides free water but no electrolyte • Peripheral IV can only administer 10% dextrose or less • Higher than 10% dextrose must go through central line