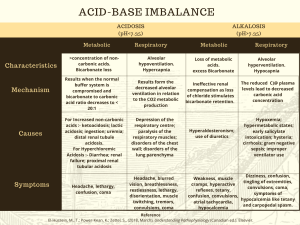
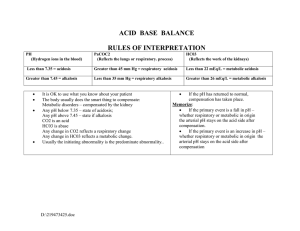
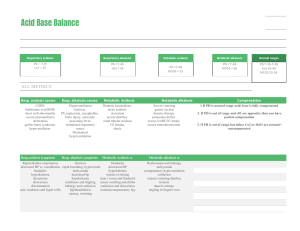
Acid-Base Balance and Arterial Blood Gases The body maintains a balance between acids and bases by the interplay between: Acid Production Acid Buffering Of Carbonic acid (H2CO3) and Metabolic acids (any acid that is not carbonic acid) Acid Excretion Via buffers - pairs of chemicals that work together to maintain normal pH of body fluids; primary regulator Acids release hydrogen (H+) ions The more H+ present = more acidic Bases (alkaline substances ) take up H+ ions Degree of acidity = pH pH 1.0 = very acidic; pH 14.0 = very alkaline (base); pH 7.0= neutral Acidosis Acid-Base Imbalance Results from an alteration in the ratio of acids and bases Signifies an underlying health problem Classified as either respiratory or metabolic Respiratory - from retention or excess of CO2 which affect H2CO3 (carbonic acid) levels Metabolic - deals with HCO3 (bicarbonate) levels Via lungs (excrete carbonic acid) and kidneys (excrete metabolic acids) ABG values provide objective info about a patient's acid-base status and underlying cause. Respiratory Acidosis - A condition where the blood is too acidic 2 Types Alkalosis What: Excess carbonic acid (H2CO3) Cause: Hypoventilation/Bradypnea (<12 bpm) due to: Mechanical hypoventilation COPD Pulmonary edema Sedatives Chest wall abnormality Severe pneumonia Respiratory muscle weakness Atelectasis Labs: pH < 7.35 CO2 > 45 Sign/Symptoms: RR < 12 bpm Headache Lethargy Coma Confusion Low BP Dizziness Warm, flushed skin Compensation: Kidneys conserve bicarbonate and secrete H+ into urine Oxygen supplementation Encourage deep breathing Suction if pneumonia Monitor K+ levels for hyperkalemia May need endotracheal intubation Bronchodilators Antibiotics Respiratory Alkalosis 2 Types ↓carbonic acid (H2CO3) (↓CO2) ↑ Metabolic Alkalosis - bicarbonate (HCO3) pH = Respiratory Acidosis Treatment/Interventions ↓ Metabolic Acidosis - bicarbonate (HCO3) pH = A condition where the blood is too basic (alkaline) ↑carbonic acid (H2CO3) (↑CO2) Lab Values to Know! pH CO2 HCO3 Acidic Normal Alkaline < 7.35 7.35 - 7.45 > 7.45 22 - 26 > 26 > 45 < 22 35 - 45 < 35 Respiratory Alkalosis What: Deficit of carbonic acid (H2CO3) Cause: Hyperventilation/Tachypnea (<20 bpm) due to: Anxiety, fear, pain, exercise, fever Stimulated respiratory center (e.g. stroke, brain injury) Liver failure Mechanical hyperventilation Labs: pH > 7.45 Treatment/Interventions CO2 < 35 Instruct on rebreathing into Sign/Symptoms: paper bag and re-breather mask RR > 20 bpm Monitor K+ levels for Lethargy hypokalemia Tetany, numbness If on mech vent monitor for Dysrhythmias hyperventilation Muscle cramps, N/V +Chvostek's sign Compensation: Kidneys secrete bicarbonate Acid-Base Balance and Arterial Blood Gases Metabolic Acidosis Metabolic Alkalosis What: Loss of bicarbonate or acid accumulation due to: acid production OR acid excretion OR Loss of bicarbonate Cause: To determine cause, look at anion gap: ↑ ↓ Anion gap: The difference between anions and cations. Usually calculated by MD; normal = 10 - 14 mEq/L High Anion Acidosis (>14 mEq/L): DKA, lactic acidosis, aspirin toxicity, renal failure, shock Normal Anion Acidosis (10 - 14 mEq/L): diarrhea, ostomies or fistula drainage, Diamox (diuretic) Labs: Treatment/Interventions pH < 7.35 HCO3 < 22 Monitor for need to intubate Sign/Symptoms: Monitor K+ levels Kussmaul respirations Check neuro status Confused, weak IV insulin if DKA BP Bicarbonate may be needed N/V May need dialysis ↓ Compensation: Kidneys will attempt to excrete excess acid and lungs will CO2 excretion (Kussmaul respirations) 1 4 Steps of ABG Interpretation Determine if values are normal/acidic/alkaline pH 2 ↑ CO2 HCO3 Acidic Normal Alkaline < 7.35 7.35 - 7.45 > 7.45 22 - 26 > 26 > 45 < 22 35 - 45 < 35 Analyze CO2 to see if problem is respiratory ↑CO2 + ↓pH = respiratory acidosis ↓CO2 + ↑pH = respiratory alkalosis R O M E espiratory pposite etabolic qual What: Excess bicarbonate due to: Loss of acid Gain in bicarbonate Cause: Hypokalemia Vomiting Excess NaHCO3 intake Nasogastric suctioning Mineralocorticoid use Diuretics Labs: Treatment/Interventions pH > 7.45 HCO3 >26 Depends on cause: Sign/Symptoms: Vomiting: give Lethargy antiemetic Confusion Diuretics: discontinue Headache Diamox may be ordered Tachycardia Monitor: Dysrhythmias K+ and Cl- levels N/V For signs of respiratory Tetany distress Tremors Tingling in fingers/toes Hypoventilation Compensation: Kidneys excrete bicarbonate and respiratory rate slows (hypoventilation) 3 Analyze HCO3 to see if problem is metabolic ↑HCO3 + ↑pH = metabolic alkalosis ↓HCO3 + ↓pH = metabolic acidosis 4 Determine if patient is compensating or not Look at the component (CO2 or HCO3) that is NOT the cause of the primary disturbance If that component is moving in the opposite direction, the body is attempting to compensate; example: pH 7.33 (acidic) respiratory acidosis CO2 55 mmHg (high) HCO3 36 mEq/L (high/alkalotic) = compensation ROME Method for ABG Interpretation If the CO2 and pH levels are opposite = Respiratory If the HCO3 and pH levels are the same = Metabolic